Russia completes early trials of second potential COVID-19 vaccine: Ifax
Change Size
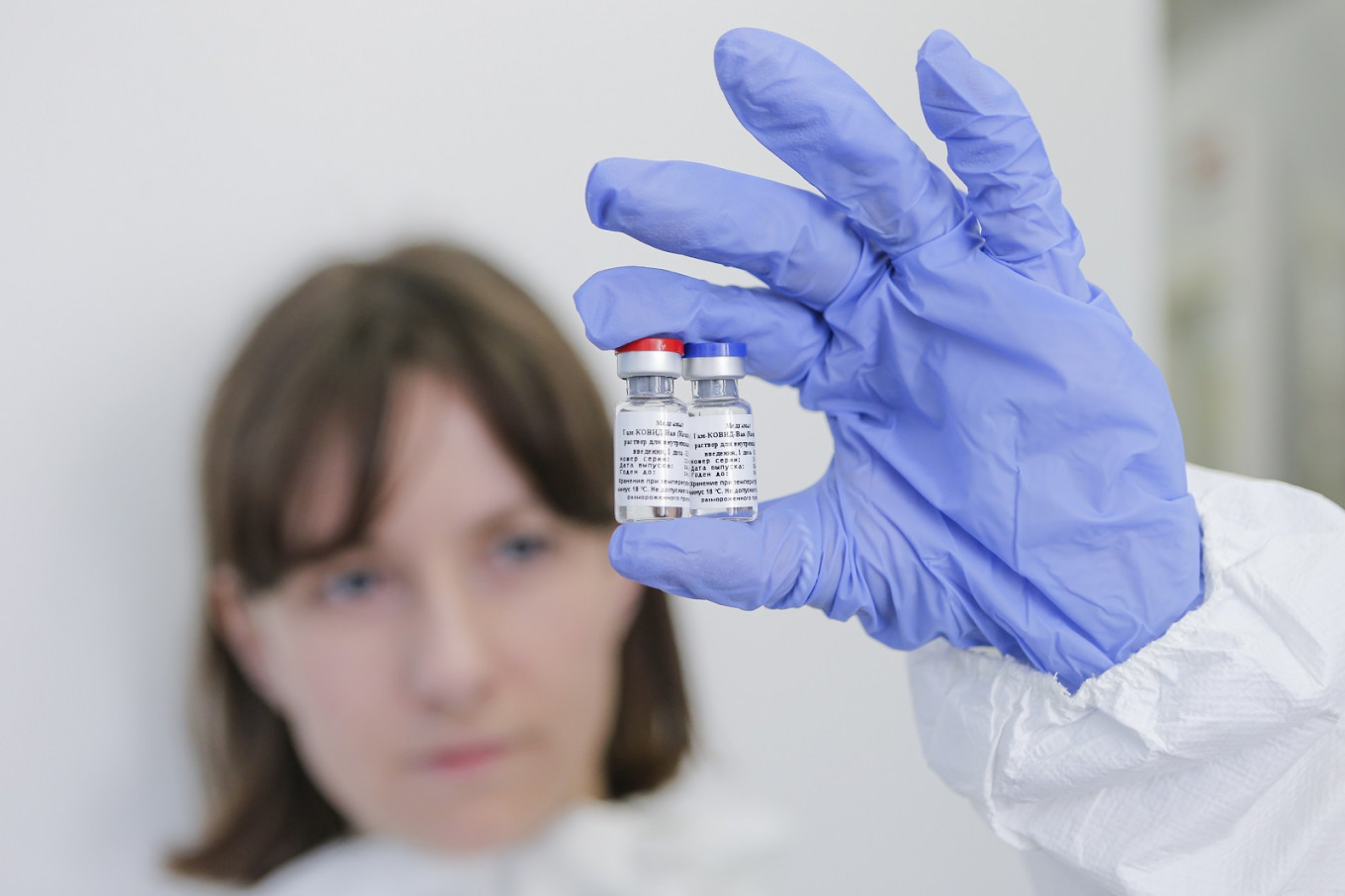 This handout picture taken on Aug. 6, 2020 and provided by the Russian Direct Investment Fund shows the vaccine against the coronavirus disease, developed by the Gamaleya Research Institute of Epidemiology and Microbiology. Siberia's Vector virology institute on Tuesday completed early-stage human trials, known as Phase II, of a second potential Russian vaccine against COVID-19, the state consumer safety watchdog was cited by the Interfax news agency as saying. (Russian Direct Investment Fund/AFP/File)
This handout picture taken on Aug. 6, 2020 and provided by the Russian Direct Investment Fund shows the vaccine against the coronavirus disease, developed by the Gamaleya Research Institute of Epidemiology and Microbiology. Siberia's Vector virology institute on Tuesday completed early-stage human trials, known as Phase II, of a second potential Russian vaccine against COVID-19, the state consumer safety watchdog was cited by the Interfax news agency as saying. (Russian Direct Investment Fund/AFP/File)
S
iberia's Vector virology institute on Tuesday completed early-stage human trials, known as Phase II, of a second potential Russian vaccine against COVID-19, the state consumer safety watchdog was cited by the Interfax news agency as saying.
Russia registered its first vaccine candidate, developed by Moscow's Gamaleya Institute, in August. Late-stage trials of this vaccine, due to involve 40,000 participants, were launched last week.
Human trials of the second potential COVID-19 vaccine, a peptide-based jab, began on July 27 and involved a group of 100 volunteers, Interfax cited watchdog Rospotrebnadzor as saying.
"Today ... the final group of 20 volunteers was released from hospital," said in a statement. "All 100 volunteers were vaccinated with two doses and have completed a 23-day monitoring period in hospital. The volunteers are feeling good."
Results are due to be published on Sept. 30, Interfax said.









