COVID-19 vaccines – A case for being cautiously optimistic?
There is currently a great sense of optimism around vaccines, but the perfect vaccine could remain elusive.
Change Size
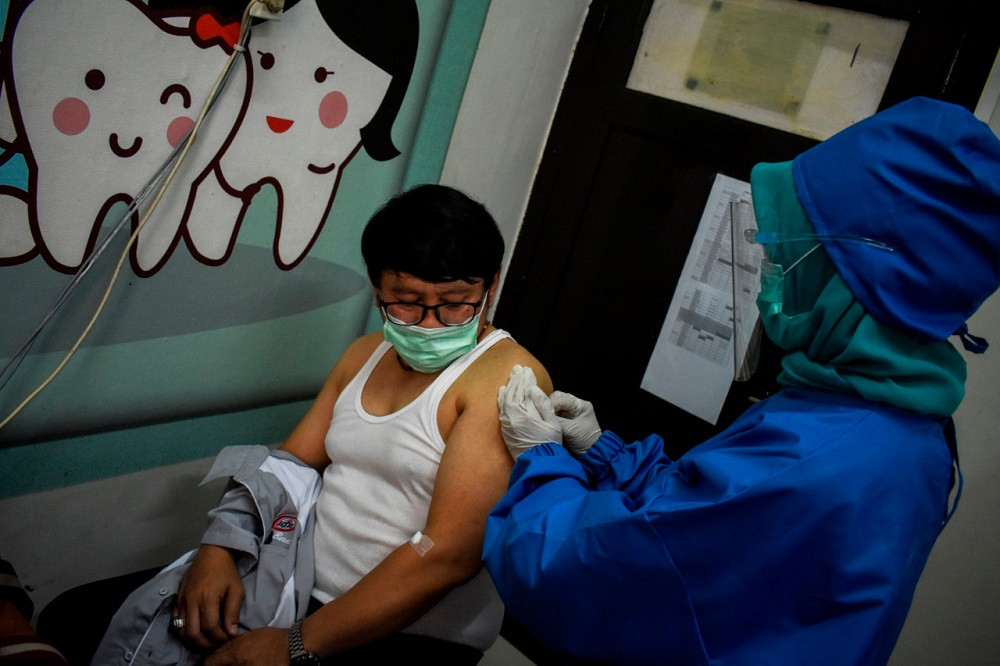 Human trial: A volunteer receives an injection of a COVID-19 vaccine candidate during a phase III trial in Bandung, West Java, on Aug. 14. The trial is being conducted on more than 1,600 healthy people in five areas across the West Java capital. (Antara/Prima Mulia)
Human trial: A volunteer receives an injection of a COVID-19 vaccine candidate during a phase III trial in Bandung, West Java, on Aug. 14. The trial is being conducted on more than 1,600 healthy people in five areas across the West Java capital. (Antara/Prima Mulia)
E
ight months into this pandemic, the number of confirmed cases of COVID-19 has exceeded 23.8 million, with over 815,000 deaths globally and no sign of levelling is on the horizon. As reliable and effective therapeutic interventions remain elusive, hopes for a return to normal life now hinge on the race to develop an efficacious vaccine.
However, vaccines have for long been a controversial topic, even more so since the advent of social media, which can rapidly fuel the spread of factual, anecdotal and downright incorrect information. Now more than ever, it is critical to ensure transparent information around vaccine development and testing.
While some people believe that natural immunity is sufficient and adequate, the risks associated with natural immunity will be much higher than those arising through vaccination. Most people develop a spectrum of mild symptoms, but infection can in some cases cause people’s immune system to overreact, resulting in inappropriate inflammation leading to lung and other organ damage, not to mention the potential long-term implications of infection now referred to as “long COVID” symptoms.
Vaccines are preventive interventions – that is – they educate the immune system to recognize a specific pathogen (germ) so that it can mount an appropriate response to prevent infection or disease upon exposure. Simply put, vaccines produce immunological memory against a pathogen, and some vaccine platforms have been optimized to tip the balance toward an immune response stronger than natural infection.
Traditionally, vaccines have been prepared from a weakened or inactivated version of the pathogen but, as our understanding of immune responses has improved, more elegant approaches have been developed. It is fortunate that the pandemic has struck at a time when multiple technologies are available – so much so, that in eight months, scientists have come up with over 150 different vaccine candidates globally. Though not all of them will make it to the end – the knowledge generated from this collective effort will be invaluable. Vaccine development requires synergy between academics, business and the government (ABG). Research institutes and academics play important roles in studying the pathogen and the disease it causes, both of which are important for designing the candidate that industry can take to the clinic and into production.
The government’s role is as a regulatory control and “push and pull” actor that creates the necessary framework for vaccine deployment. Research institutions, small and large biotech, and big pharma companies can all play roles in the race to develop a vaccine. Though, for this to take place rapidly, unprecedented levels of funding are required.
New vaccine platform technologies can offer tremendous promise for conquering infectious disease threats in a more rapid, cost-effective, and technologically elegant manner, but traditional vaccinology remains a major driver of vaccine development and need not be wholly abandoned.
Traditional vaccines are developed based on the old art of either weakening or inactivating large batches of virus preparations generated by growing the virus using specific cells under controlled conditions. At this stage there are two Chinese companies with traditional vaccines in phase III: Sinopharm and Sinovac. Since the production of the vaccines utilizes well-known and fine-tuned processes, these could – on the back of successful phase III trials – potentially be on the market in the first quarter of 2021. One such trial (NCT04508075) is evaluating Sinovac’s product in Bandung, West Java, among 1,620 healthy volunteers (aged 18-59 years) and is expected to assess whether two doses of vaccines can prevent either infection or the incidence of disease within the six-month period post-vaccination. These studies are lengthy and should be conducted following good clinical practice.
The next generation of vaccines can be produced using more elegant approaches. Genetic vaccines use synthetic copies of the coronavirus’ own genetic information (e.g. RNA) to stimulate an immune response. When injected, it tricks the cell’s machinery into making virus antigens and the immune system then reacts to those antigens. This type of vaccine is theoretically simpler and cheaper to produce and two companies – Moderna (United States) and BioNTech (Germany)/Fosun Pharma (China)/Pfizer (US) – have entered phase III clinical trials in the US for RNA vaccine candidates. To this day no product exists on the market based on this platform.
Viral vector-based vaccines use a virus to deliver coronavirus genes into cells and stimulate an immune response. Major players include Oxford (United Kingdom)/Astra Zeneca (UK) and Cansino (China). Both have developed adenoviral vaccines currently in phase III clinical trials. No viral-based vaccine exists on the market to date.
Protein-based vaccines use a coronavirus protein, identified based on immunological studies, or a protein fragment to provoke an immune response. Most players are focusing their efforts on the Spike protein. The protein generally requires formulation with an adjuvant (immune stimulating) component to ensure that it stimulates a strong immune response. Several players are developing this type of vaccine including the Eijkman Institute in Jakarta, but none has started phase III trials. Other protein-based vaccines already exist on the market.
There is currently a great sense of optimism around vaccines, but the perfect vaccine could remain elusive. Vaccine efficacy can vary between products. The measles vaccine has an efficacy of 95-98 percent while the flu vaccine is 20-60 percent effective. The efficacy of current COVID-19 vaccine candidates will not be known until phase III trials are complete, and how well the vaccines work in the real world, rather than under the strict regimen of clinical trials, will take even longer to understand. Until then, introduction of the vaccine might still need to be supported by social distancing and mask wearing for some time.
Beyond the difficulties of the discovery, vaccine development must overcome the challenges of technical translation so production can be scaled up and run reproducibly in a manufacturing environment, in adherence with stringent regulatory requirements and at a sufficient scale.
Finally, once a vaccine is developed, complex questions on how the vaccine is distributed, how people can access it and on what basis will arise. The question will be: can fair and equitable access to the vaccine be guaranteed?
***
Ines I Atmosukarto is CEO and chief scientific officer of Lipotek Pty Ltd, an Australian niche biotech company with an interest in nanoparticle-based vaccine technology. Neni Nurainy is project integration manager of the research and development division at PT Bio Farma. The views expressed are their own.