Bio Farma to produce more than 16 million doses of COVID-19 vaccine per month
Indonesia needs 340 million doses of vaccine within a year to vaccinate around 60 percent of the population.
Change Size
 President Joko "Jokowi" Widodo surveys state-owned enterprises’ (BUMN) readiness and facilities to produce vaccines on a working visit to the city of Bandung on Aug. 11. (Biro Pers Sekretariat Presiden/Kris)
President Joko "Jokowi" Widodo surveys state-owned enterprises’ (BUMN) readiness and facilities to produce vaccines on a working visit to the city of Bandung on Aug. 11. (Biro Pers Sekretariat Presiden/Kris)
S
tate-owned pharmaceutical holding company PT Bio Farma plans to produce 16 to 17 million doses of COVID-19 vaccine per month, corporate secretary Bambang Heriyanto said on Monday.
Indonesia needs 340 million doses of vaccine within a year to vaccinate around 60 percent of the population.
The Indonesian government has secured deals to procure approximately 300 million doses of COVID-19 candidate vaccines from various developers, including China’s Sinovac Biotech Ltd. and China National Pharmaceutical Group (Sinopharm) as well as United Kingdom-based AstraZeneca.
Bambang explained during a virtual talk show hosted by the National Disaster Mitigation Agency (BNPB) that Bio Farma had a capacity to produce 250 million doses of vaccine annually. The company planned to produce 16 to 17 million doses per month, depending on the supply from China’s Sinovac Biotech Ltd.
Bio Farma has teamed up with Sinovac to launch Phase III clinical trials of the candidate vaccine developed by the Chinese company in Bandung, West Java.
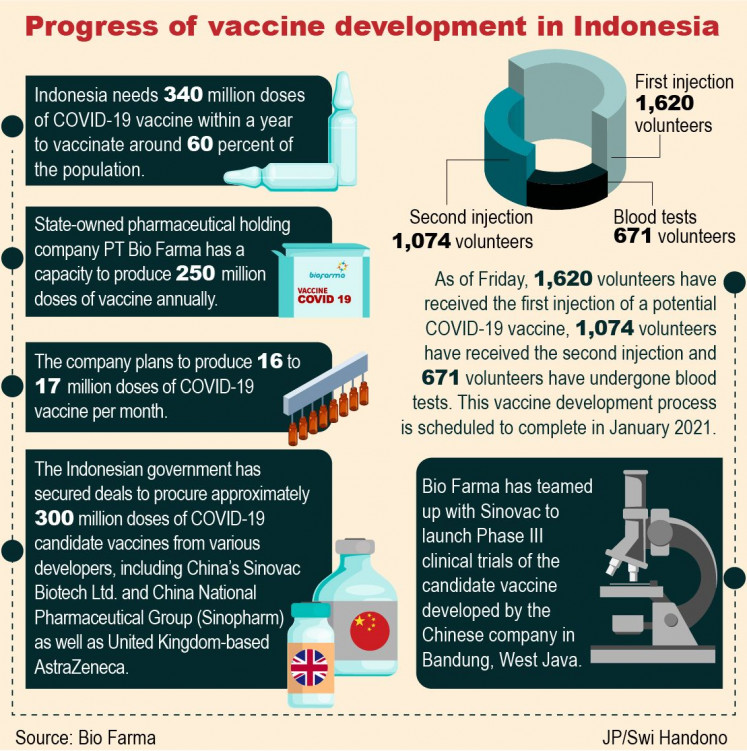
Bambang said that, as of Friday, 1,620 volunteers had received the first injection of the COVID-19 vaccine, 1,074 volunteers had received the second injection and 671 volunteers had undergone blood tests after receiving the second injection.
Bambang added that they hoped to complete the entire development process in January.
“We are going to start producing once we get the license from the Food and Drug Monitoring Agency [BPOM],” he said.
Read also: COVID-19 vaccine to be priced at Rp 200,000 per dosage: Bio Farma
Last week, Bio Farma confirmed that the COVID-19 vaccine would be priced at around Rp 200,000 (US$13.57) per dosage when it becomes available.
Bio Farma president director Honesti Basyir said the price was based on a recent email from Sinovac Biotech, the Chinese COVID-19 candidate vaccine producer, explaining that the price of the potential vaccine was based on the investment put into the Phase III clinical trials and efficacy test.
The coordinator and spokesperson of the national COVID-19 task force, Wiku Adisasmito, said the vaccine would be available once all the clinical trials showed that it was safe and effective.
However, Wiku said COVID-19 vaccines were not the only reassurance for the country to end the pandemic, explaining that vaccines only served as a form of medical intervention to boost people’s immunity during the pandemic.
While waiting for the COVID-19 vaccine, Wiku urged the public to comply with the “3M” health protocol of mask-wearing, handwashing and physical distancing.
He also recommended that people consume healthy food and exercise to boost their immunity. (jes)
Editor’s note: This article is part of a public campaign by the COVID-19 task force to raise people’s awareness about the pandemic.









