Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
View all search resultsEfficacy and effectiveness determine quality of COVID-19 vaccine: Task force
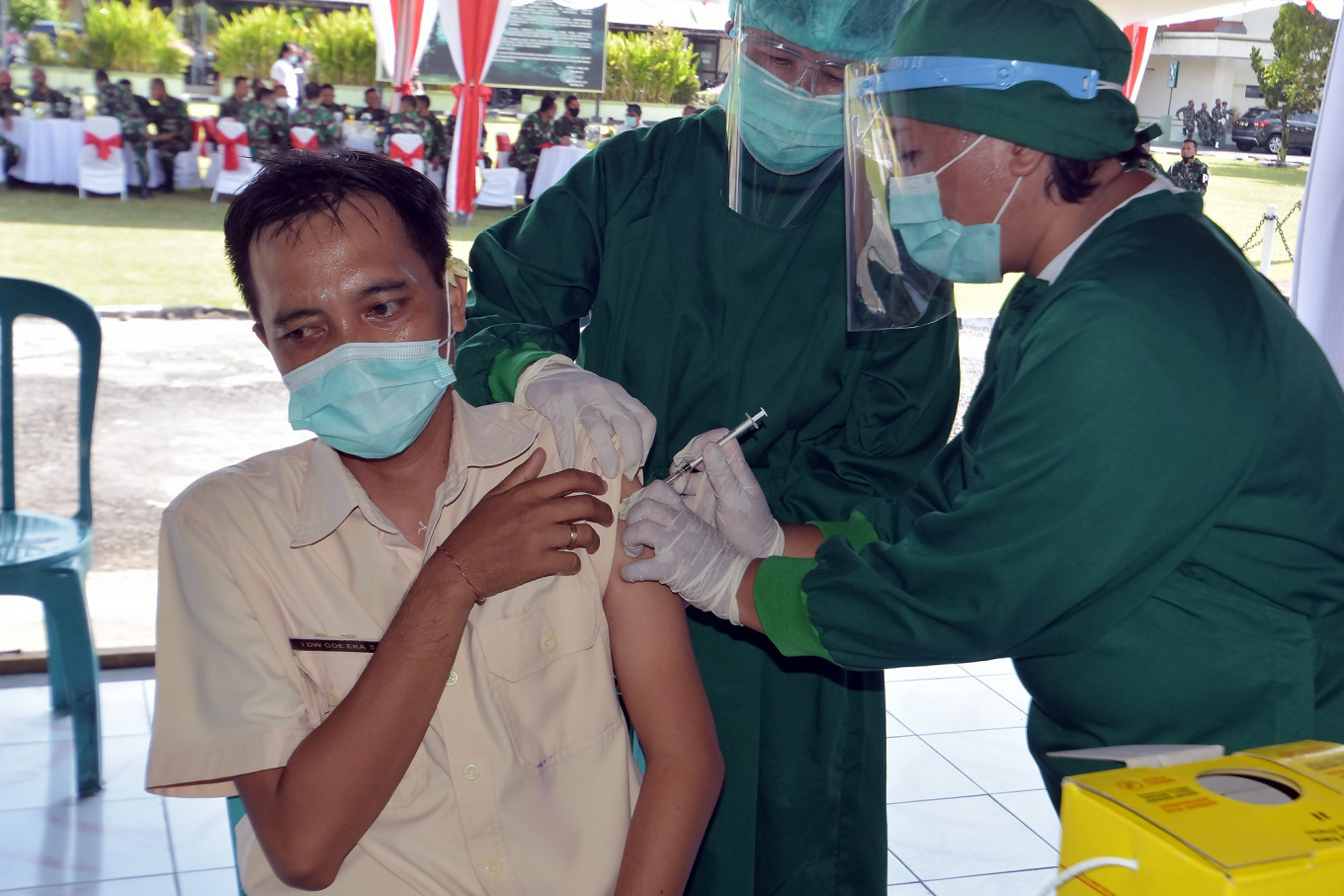
To measure its efficacy, the vaccine has to pass a series of clinical trials and be tested on a limited population.
Change text size
Gift Premium Articles
to Anyone
Efficacy and effectiveness are among important factors that determine the quality of a vaccine, COVID-19 national task force spokesman Wiku Adisasmito has said.
“These aspects play an important role in measuring the benefits of COVID-19 control,” Wiku said as quoted by kompas.com on Thursday.
Wiku explained efficacy meant the vaccine’s ability to prevent the transmission of the coronavirus and to keep the pandemic under control. To measure its efficacy, the vaccine has to pass a series of clinical trials and be tested on a limited population.
Meanwhile, the effectiveness is the vaccine’s ability to protect the public from the disease. There are many factors that affect a vaccine’s effectiveness, such as its recipient’s age, comorbidities, infection history, as well as the vaccine duration.
The second factor is the vaccine’s own characteristics, namely its composition, injection method or whether it has been activated.
“The third factor is whether the vaccine’s strain matches the virus strain,” he added.
To measure the vaccine’s effectiveness, the manufacturer should study its surveillance data to see its development and to monitor its effect on the recipients.
Read also: Indonesia to cover only a third of COVID-19 vaccine recipients
Recipients’ immunization and medical records will be studied as well to see if there are other aspects that will affect the individual’s health condition.
The vaccine’s efficiency could be seen from how its production cost could reduce financial burdens caused by the disease.
State-owned pharmaceutical company PT Bio Farma previously announced that it would allocate 3 million doses of a COVID-19 vaccine made by China’s Sinovac Biotech for health workers in the country.
Bio Farma president director Honesti Basyir explained that the vaccine would be delivered from China in two phases. The first phase consists of two shipments, the first of which has arrived in the country with 1.2 million doses of the vaccine. The second shipment, which contains 15 million doses of the vaccine, is expected to arrive before the end of December.
The second phase of delivery will also be in two phases. The first shipment will contain 3 million doses of the vaccine, 1.8 million of which will come in a single-dose package in January next year. The second shipment, which will contain 30 million doses of the vaccine, will also be delivered at the beginning of 2021.
“Bio Farma will focus on storing the vaccine and preparing for its distribution after obtaining a license for use from the BPOM,” Honesti said in a statement on Tuesday. (dpk)
Editor’s note: This article is part of a public campaign by the COVID-19 task force to raise people’s awareness about the pandemic.