Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
View all search resultsScientists extend volunteer registration for COVID-19 vaccine trial
Registration to remain open until 1,620 subjects injected.
Change text size
Gift Premium Articles
to Anyone
T
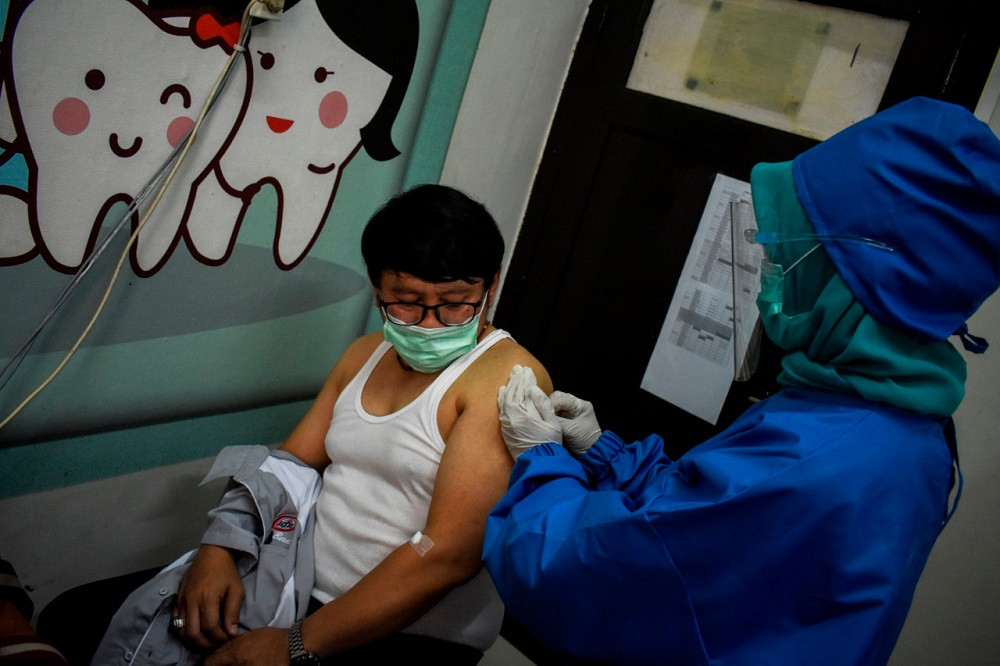
he team of scientists conducting phase III clinical trials for a COVID-19 candidate vaccine developed by Chinese biopharmaceutical company Sinovac Biotech has announced that it will extend volunteer registration for the vaccine trials.
Team spokesperson Rodman Tarigan said 2,300 people had volunteered for the trial, exceeding the 1,620 initially targeted.
"We decided to extend the registration because we're worried some volunteers will not show up. It's also to anticipate volunteers who don't meet our criteria," Rodman told The Jakarta Post on Tuesday.
He said registration would remain open until 1,620 subjects had been injected with the candidate vaccine.
The clinical trials are conducted in six different locations in the West Java provincial capital of Bandung, including four community health centers (Puskesmas) that are respectively located in Sukapakir, Garuda, Ciumbuleuit and Dago and in Padjajaran University’s (Unpad) hospital and health center.
Rodman explained there were three stages of the trials. The first stage is called V0, where volunteers would be tested for COVID-19 using a polymerase chain reaction test and receive a clear explanation about the process from doctors. They would then sign an agreement to state their ability to be test subjects.
Read also: 'It's for humanity': Indonesians step up to volunteer in vaccine trials
In the second stage, which is called V1, subjects who tested negative for COVID-19 would be injected with the candidate vaccine. Two weeks later, in the third stage called V2, the subjects would receive the second injections.
The process will end six months after the first visit. During the period, blood samples will be taken three separate times.
The subjects are also required to report their health condition after the injections.
According to Rodman, since the start of the clinical trials, 248 people had been injected with the candidate vaccine, 21 of whom had received second injections.
"In general, they only reported sores around the injection area, without any fever. It's a very common reaction," he said.
Head of Unpad’s clinical trial team, Kusnandi Rusmil, said the team would focus on analyzing 540 subjects' blood samples to assess the vaccine’s efficacy, immunogenicity and safety.
"The remaining 1,080 samples will be used to discover the side effects of the vaccine," Kusnandi said.
"We expect to finish the trial for the 540 volunteers before December," he added. (nal)