The fast-moving Omicron variant, which the World Health Organization calls a variant of concern, has spread to Indonesia, with health authorities reporting 96 new cases on Monday, bringing the total tally of Omicron cases to 506 as of earliest January, 2022.
The emergence of the ultra-contagious Omicron has clouded forecasts for the end of COVID-19 despite the declining trend in the cases in Indonesia. It has confirmed experts’ view that the SARS-CoV-2 coronavirus that causes COVID-19 will mutate periodically, resulting in different variants of the virus. Given that a mutation is expected to occur once every four or six months, we should learn to live with the virus instead of ignoring it. This means that preventive measures such as testing and therapeutics should be considered as part of the new normal.
Not all types of vaccines are reportedly effective to prevent Omicron, meaning that everyone needs a COVID-19 booster as only a certain set of vaccines will offer stronger immunity.
Therefore, it raises questions not just about the current level of vaccinations, but also the level of boosters. More boosters are likely needed annually going forward. Indonesia will begin giving COVID-19 booster shots to the general public from Wednesday.

Indonesia has been hit hard by COVID-19 and continues to struggle to cope with the health crisis that has had a severe impact on the population socially, economically and psychologically. The government, tasked with handling this crisis, must take into account not just healthcare considerations, but also economic and social ones.
But vaccination alone is not enough to restore back to normal activities. Many can transmit the virus when in pre-symptomatic or asymptomatic situations. The vaccination movement should still be supported by routine massive testing as a precautionary measure for the spread of the COVID-19 virus. Testing becomes very critical so that everyone can stay active safely and become a new habit lifestyle.
Epidemiologist Dicky Budiman from Griffith University, Australia, warned that unless there were incredibly impactful measures such as intensive and frequent mass testing of up to 3 million individuals per day, the implementation of regional quarantine and the acceleration of the vaccination drive, Indonesia is predicted to be the last country to emerge from the COVID-19 pandemic, BBC.com reported.
Indonesia’s economy has been severely impacted: millions of people are facing hardship with a total of 9.1 million people unemployed as of November 2021, according to Statistics Indonesia (BPS).
Meanwhile, the vaccination drive, which kicked off in January 2021, remains in progress despite a slow start, largely due to the lack of available vaccines and human resources to support the program. As of earliest January, 2022, about 117 million people have been fully vaccinated, which is about 42.9 percent of the targeted 208.205 million eligible population.
With Indonesia experiencing challenging economic woes that have ultimately affected people’s purchasing power, breakthrough efforts must be made to help those in need. As vaccine availability remains irregular, frequent mass testing takes center stage. Tests, however, must not only be set at a low price, but also be fast and accurate. The COVID-19 testing kits currently available are either costly or impractical — or both.

Affordable frequent mass testing is critical
Global expert epidemiologists and virologists agree that COVID-19 will likely remain a part of our world for many years to come. To achieve herd immunity, i.e. a large portion of a community (the herd) becoming immune to a disease, a high level of vaccination coverage is needed with a vaccine that proves effective enough to limit the global spread of the infection to a controllable level.
However, recent studies indicate that while COVID-19 vaccines protect against the virus, their effectiveness wanes within months. A comprehensive United Kingdom study from UK Technical Briefing of COVID-19 cases has shown that people who are vaccinated have good immunity initially, but quickly become more vulnerable to the fast-spreading Omicron variant. What seems to be clear is that it is unlikely that a vaccinated and boosted individual is immune from becoming infected and consequently, is still able to transmit the virus.
Epidemiologists from renowned institutions such as Harvard’s Wyss Institute of Biologically Inspired Engineering are, therefore, calling for a clear shift in strategy. They are advocating for low-cost, frequent mass testing that can be as effective as the vaccine approach in interrupting transmission.
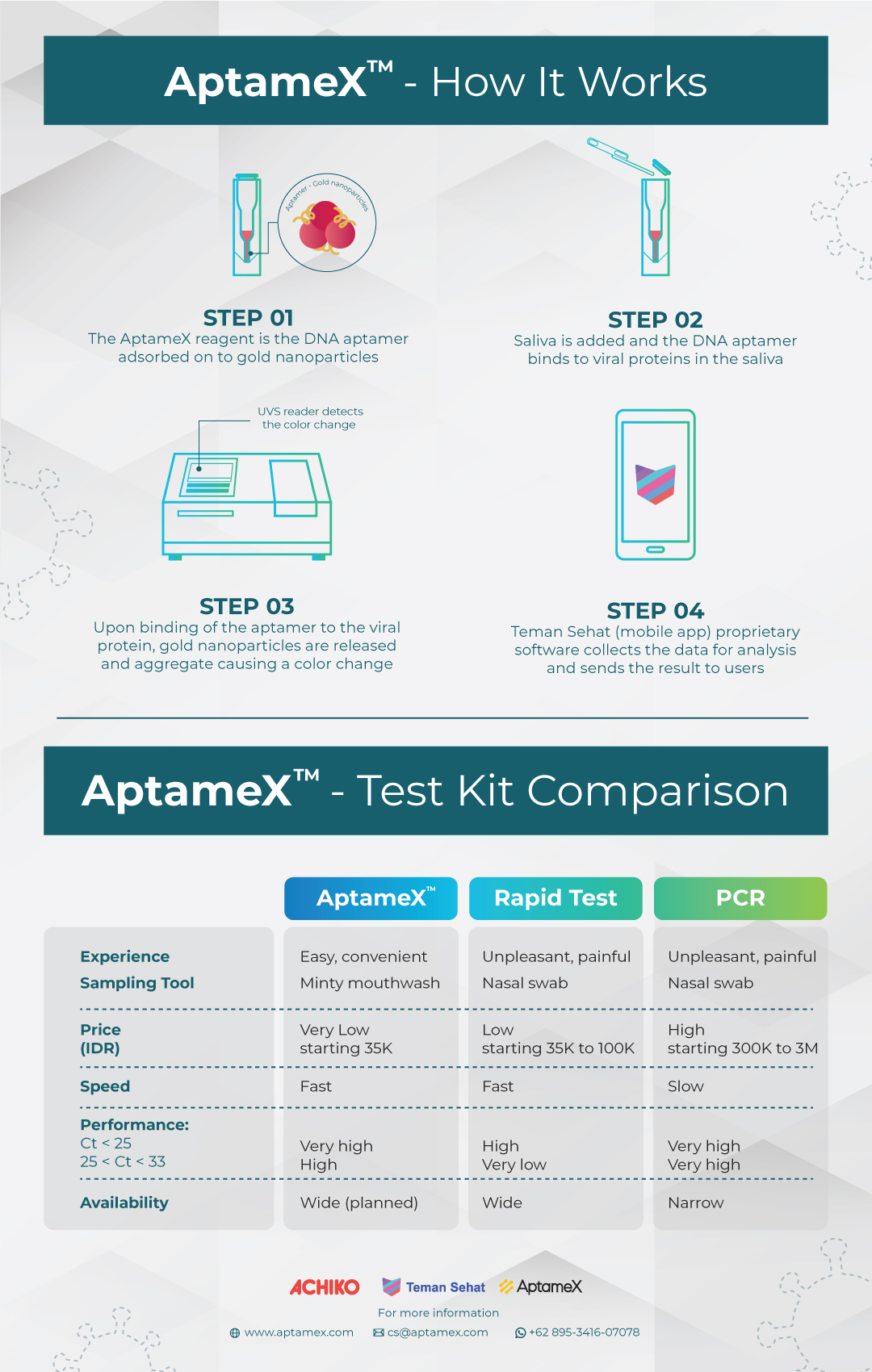
Essentially, there are currently three main testing formats, namely reverse transcription polymerase chain reaction (RT-PCR), rapid lateral flow antigen tests and antibody testing. RT-PCR test achieves accuracy at low viral loads allowing for a high degree of certainty in the diagnosis of an individual being infected. Typical viral load detected by PCR test is at Ct value 28-29 but Ct value 25-33 is the problem area. Rapid test fail at this value and PCR test are typically ineffective.
Providing an accurate testing solution that can fulfill the requirement for frequent mass testing at a price point that is accessible to all is critical.

AptameX: A new paradigm
Team in Spain, Indonesia, Philippines, Australia, and Thailand with lot of the work done in Indonesia, have made an important breakthrough and developed a completely new COVID-19 diagnostic tool. Swiss healthtech company Achiko AG (Achiko) developed the AptameX test kit as a solution to this mass testing conundrum and showing high precision result at Ct value 25 to 33. AptameX is neither an RT-PCR test nor is it a rapid lateral flow antigen test; rather, it is new chemistry involving short sections of DNA aptamers that bind to the spike protein of the SARS-CoV-2 virus.
For those seeking an accurate testing solution in a practical and affordable way, AptameX is the right choice. It is fast, safe and non-invasive, providing results in 15 minutes; matching all lateral flow rapid tests, at an affordable price. Users of AptameX can have easy access to the results because it is connected to a mobile passport application (Teman Sehat) that displays results as a digital passport. In addition, AptameX can be synthetically mass-produced with ease.
To make AptameX’s diagnostic kits readily available at a price point that is accessible to all, Achiko has partnered with partially state-owned pharmaceutical company PT Indorama Tbk.
The development of AptameX was made possible by the work of Michael Edel PhD, director and founder of Spain-based Regenacellx.sl, a company primarily focused on regenerative medicine applications to treat aging and related indications.
“The breakthrough technology is rooted in our efforts to create a platform enabling the creation of aptamer-based applications. AptameX is a response to the COVID-19 pandemic, to try and help people to return to normality,” Edel told The Jakarta Post.
“The fact that the technology is now approved for use in Indonesia makes me very proud. Indonesia, especially Pekanbaru [Riau] and Jakarta, holds a special place in my heart because my father was born in Java. My grandfather, Jan Edel, spoke Indonesian and was sent to Indonesia in 1938 to support Indonesians to understand and navigate Dutch law and regulations. In 1942, he was conscripted to fight the Japanese and died in the Pekan Baru Railway line camps in 1945, having worked alongside thousands of Indonesians,” said Edel, recounting his family history.

AptameX: A solution for today – and tomorrow
On closer inspection, the case for testing proves even more compelling. Achiko’s CEO, Steven Goh, explains: “Vaccination programs offer the promise of curbing COVID-19, yet governments and businesses are increasingly accepting what epidemiologists have long warned: The pathogen will circulate for years, or even decades, leaving society to coexist with the virus, much as it does with other endemic diseases such as the flu and measles.
“We are proud and excited to receive product approval and registration from the Health Ministry after months of technology development in Spain and Indonesia. Bringing AptameX to market with Indofarma is an immense achievement for us.
“The combination of AptameX and Teman Sehat provides entire communities with an affordable and easy-to-use test as a screener. This directly empowers them in their fight against COVID-19 and all its variants. We look forward to increased distribution of the AptameX test kits throughout Indonesia and beyond later this year.” Arief Pramuhanto, president director of PT Indofarma Tbk in Indonesia, underscored the importance of having regular testing to more effectively manage the pandemic.
“Testing regularly is crucial in containing the COVID-19 virus,” said Pramuhanto. “With the populace having access to reliable testing, we can create a path that will enable us all to get our lives back as we look to control the spread of COVID-19.” Achiko’s local entity, Achiko Medika Indonesia, oversees production and distribution of its AptameX COVID-19 test kits in Indonesia. Users will be able to easily conduct testing by rinsing with a mouthwash, collecting a saliva sample and dropping it off at a partner clinic or hospital that will then deliver the test results to their smartphone in minutes through our digital passport technology platform, Teman Sehat.
“AptameX was strategically designed to utilize ultra-low-cost materials, allowing for lower production costs, which enables Achiko Medika Indonesia to initially price AptameX below Rp 50,000 [US$3.49],” said Windiaprana Ramelan, Asia senior vice president of operations of Achiko AG.
“Achiko Medika Indonesia anticipates an even lower cost of testing in the future as manufacturing and distribution efficiencies are captured.”