Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
View all search resultsNo emergency vaccine approval this year: BPOM
“We don’t have the finished goods yet, so we can’t just make guesses,” the health minister said.
Change text size
Gift Premium Articles
to Anyone
I
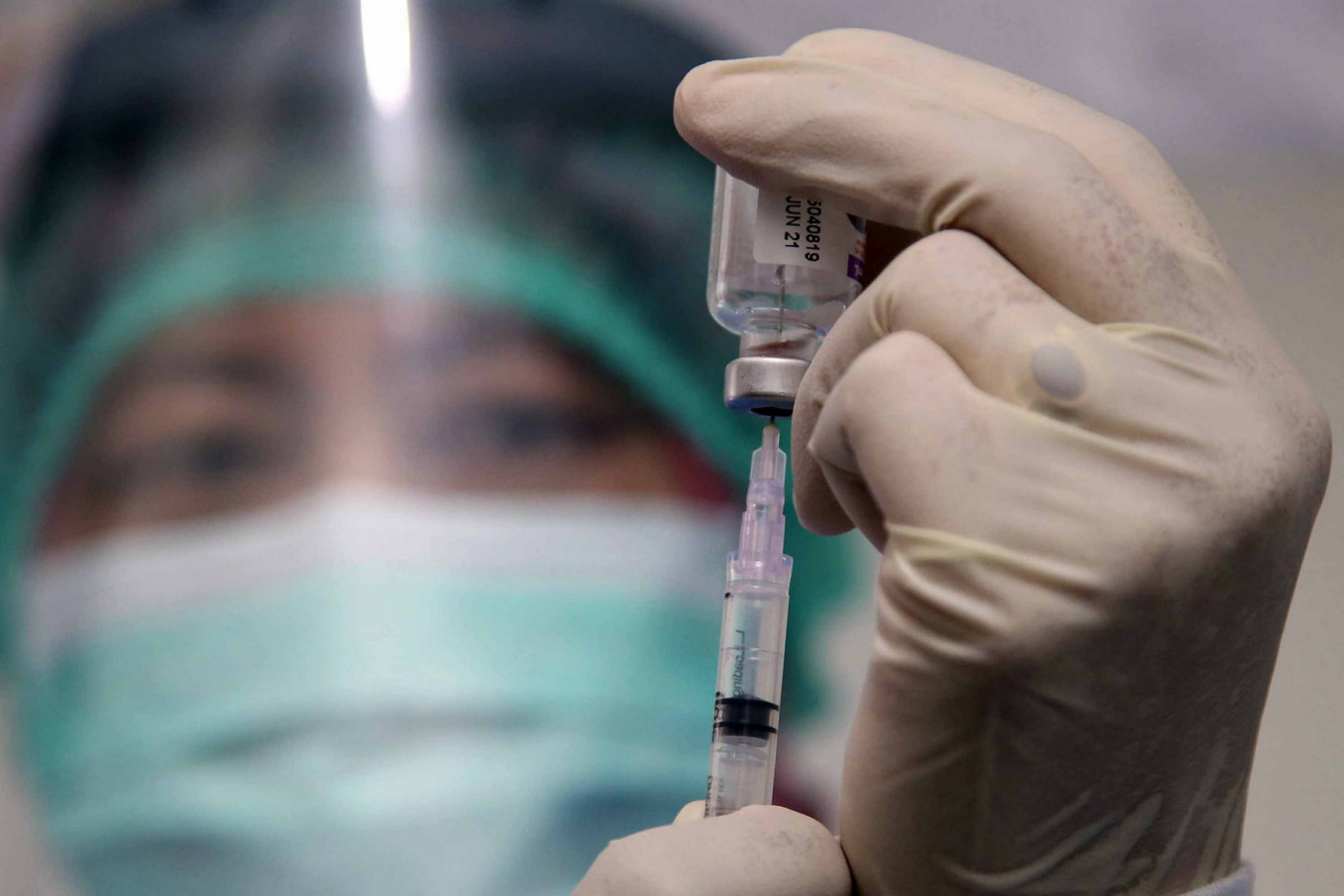
ndonesia may not be able to start its proposed mass vaccination program this year because of efficacy concerns, despite President Joko “Jokowi” Widodo’s push to fast-track the effort amid rising cases and rampant health protocol violations.
The Indonesian Food and Drug Monitoring Agency (BPOM) has said it will not authorize the emergency use of a COVID-19 candidate vaccine in December because of a lack of data on its effectiveness.
However, the agency has offered an alternative restricted vaccination program through a “compassionate use” provision, where a potential vaccine whose efficacy has not been proven may be used in a limited capacity.
BPOM head Penny Kusumastuti Lukito told lawmakers on Tuesday that during an inspection of Sinovac Biotech facilities in China, her agency had obtained data from the first two phases of the candidate vaccine’s clinical trials. The data from the final stage of testing, taking place in Indonesia, would be available in late November.
The Sinovac candidate vaccine is one of several potential COVID-19 vaccines in final-stage testing globally. Sinovac has said it is confident about the safety of the potential vaccine.
However, according to Penny, final data on the candidate vaccine’s efficacy will arrive later than expected. This information is required before the vaccine’s approval for emergency use, as agreed in a World Health Organization forum on vaccine consultation on Nov. 6.
The forum was attended by world drug regulators, including the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
An interim analysis of the clinical trials would be obtained after three months of observation, Penny said. An efficacy rate of at least 50 percent would be needed for the vaccine’s approval.
“We have explained to the President that we cannot meet the deadline for emergency use authorization in the second or third week of December,” Penny said, adding that approval could be given in the third or fourth week of January 2021.
The BPOM requested data from Brazil, which is also conducting trials on a Sinovac vaccine, but Brazil was not able to provide the data as it had not completed its trials and had no policy to release partial data early.
While waiting for the clinical trials to be completed in Indonesia, the government can access the vaccine through the WHO compassionate use provision.
Compassionate use can be granted upon request from health facilities for restricted purposes. The Health Ministry would be responsible for vaccination under the provision, and the vaccination would have to be offered voluntarily, Penny said.
Read also: Clinical trials of Sinovac's vaccine in Indonesia still on track
Under compassionate use, China had started to vaccinate health officers and military personnel, Penny added. The US had also used such provisions during Ebola and yellow fever outbreaks.
Candidate vaccine supplies secured from Chinese pharmaceutical firms Sinovac, Sinopharm and CanSino Biologics could also be used under the provision, she said. The first shipments were expected in November.
Health Minister Terawan Agus Putranto claimed that he had a grand design for vaccination, complete with a road map and technical instructions. He said the ministry had coordinated closely with the BPOM and the national COVID-19 task force.
The ministry, he added, would always uphold the principles of prudence and cautiousness that Jokowi expected. “We pray that the sooner [the vaccine arrives], the better, but it must also be safe. We don’t have the finished goods yet, so we can’t just make guesses.”
Using 246 million doses of a future vaccine, the government’s vaccination program would target 107 million people, national economic recovery committee head Budi Gunadi Sadikin said.
Budi said the government would rely on foreign vaccines because the Merah Putih (Red and White) vaccine being developed by the Eijkman Institute for Molecular Biology was not ready.
As of Tuesday, Indonesia had confirmed 474,455 COVID-19 cases and 15,393 deaths.
Daily cases have continued to rise amid serious COVID-19 protocol breaches, including recent mass gatherings of conservative Muslims to welcome the return of Islam Defenders Front (FPI) leader Rizieq Shihab from self-exile in Saudi Arabia.
While considering tougher measures against health protocol offenders, Jokowi’s administration has been planning for an eventual vaccination program.
Jokowi claimed on Tuesday that the Sinovac clinical trials in Indonesia were on schedule and that the government would follow scientific principles in the vaccination program for the safety of the community.
"We want the safety and security of the community to be the highest priority,” he said.
He said the government would vaccinate select groups of people, namely health workers, security personnel, public servants and civil servants, including teachers, as soon as the vaccines were approved for emergency use.
Indonesian Institute of Sciences (LIPI) biotechnology researcher Wien Kusharyoto said the BPOM had made the right decision to delay the emergency authorization so that concrete trial data could be obtained.
Wien added that state-owned pharmaceutical company PT Bio Farma, which is in charge of the Sinovac vaccine trials in Bandung, had not disclosed the number of trial volunteers who had been incidentally exposed to the virus, which would be used to determine the vaccine’s efficacy.
“In Indonesia, the number of volunteers is small, while the transmission rate of COVID-19 is also lower than that of other countries, such as the US, making it more difficult to detect the vaccine’s efficacy,” he said.
The Pfizer vaccine, he noted, had reported 95 percent efficacy, meaning the vaccine had prevented COVID-19 symptoms in 90 percent of volunteers who received the vaccine, compared to a control group.
Wien also commended the BPOM for revoking its emergency approval of chloroquine and its distribution permit for hydroxychloroquine as COVID-19 treatments over efficacy concerns.