Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
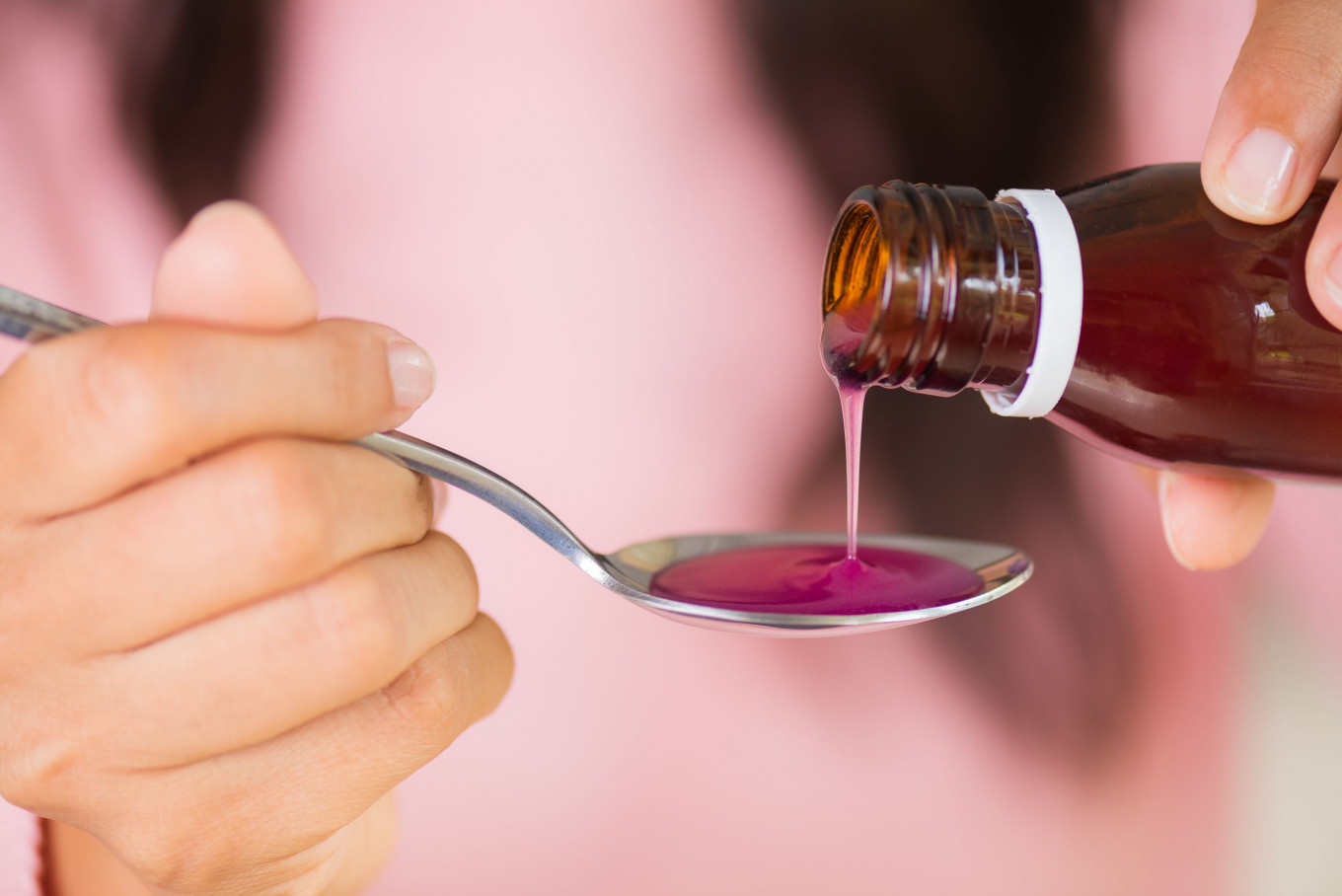
View all search resultsWHO says toxic syrup risk ‘ongoing’, more countries hit
There was an ongoing global threat posed by toxic cough syrups, the World Health Organization (WHO) told Reuters, saying it was now working with six more countries than previously revealed to track the potentially deadly children’s medicines.
Change text size
Gift Premium Articles
to Anyone
T
here was an ongoing global threat posed by toxic cough syrups, the World Health Organization (WHO) told Reuters, saying it was now working with six more countries than previously revealed to track the potentially deadly children’s medicines.
The United Nations agency has already named nine countries where tainted syrups might have been sold, after the deaths of more than 300 infants on three continents last year were linked to the drugs.
Rutendo Kuwana, the WHO team lead for incidents with substandard and falsified medicines, declined to name the six new countries the agency was working with while investigations were underway.
He warned that contaminated medicines could still be found for several years because adulterated barrels of an essential ingredient might remain in warehouses.
Cough syrups and the ingredient propylene glycol both have shelf lives of around two years.
“This is an ongoing risk,” said Kuwana.
Unscrupulous actors sometimes substituted propylene glycol with the toxic alternatives ethylene glycol and diethylene glycol because they were cheaper, several pharmaceutical manufacturing experts told Reuters.
The alternatives are more commonly used in brake fluid and other products not meant for human consumption.
The WHO’s working theory was that in 2021, when prices of propylene glycol spiked, one or more suppliers mixed the cheaper toxic liquids with the legitimate chemical, Kuwana said. He did not say where the suppliers were based, adding that obscure supply chains had made proving this difficult.
Pharmaceutical manufacturers, including those alleged to have produced the tainted syrups that have been found so far, typically source ingredients from external suppliers.
Liberia and Cameroon
Earlier this week, Nigeria’s regulator issued a warning about contaminated paracetamol syrups sold in Liberia, although no deaths have been reported there. The Nigerian regulator was testing the syrups, which were not sold in Nigeria, because Liberia had no testing facilities.
The WHO issued safety alerts last year for Indian-made products found in Gambia and Uzbekistan, and this year in Micronesia and the Marshall Islands.
It also issued an alert last year for Indonesian-made syrups that were only sold domestically. Indonesian authorities said more than 200 children were likely poisoned by these medicines.
Three Indonesian manufacturers, PT Yarindo Farmatama, PT Universal Pharmaceutical Industries and PT AFI Farma, have had their licenses revoked. A fourth, PT Konimex, said it had recalled all of the relevant products and its website says it was cleared by the government to sell new batches as of December 2022.
In January, the WHO said it was working with four other countries, Timor-Leste, Cambodia, Senegal and the Philippines, to track whether any of the tainted syrups had reached their markets.
There was no current risk to the populations in the countries the WHO had named, Kuwana said, either because contaminated medicines had been pulled from shelves or because they had never reached the market in the first place.
The countries’ governments either confirmed this, said there was only minimal risk, or did not respond to requests for comment.
The WHO said it had also offered help to Liberia and Cameroon, which recently signaled that it too might have contaminated cough syrups for sale.
Cameroon’s health regulator said in April it was investigating the deaths of six children linked to a cough syrup branded as Naturcold.
The manufacturer named on the packet is China’s Fraken Group, which did not immediately respond to requests for comment.
But the Cameroon authorities said in an alert that the medicine was bought from unauthorized sources and possibly smuggled in. They did not respond to requests for more information.
Other manufacturers identified in the current spate of incidents are largely Indian-based. Local authorities have shuttered two companies whose products have been linked to deaths:
Maiden Pharmaceuticals, which sold syrups to Gambia, and Marion Biotech, which distributed its syrups to Uzbekistan.
Naresh Kumar Goyal, the founder of Maiden Pharmaceuticals, told Reuters in December that his company did nothing wrong in the production of the cough syrup.
Marion Biotech has not responded to requests for comment.
Besides these cases, Indian-made medicines supplied to the Marshall Islands and Micronesia were recalled after Australian laboratory tests showing contamination prompted a WHO safety alert. The manufacturer, QP Pharmachem, told Reuters earlier this year that its own tests had found no issues.
The contaminated syrups in Liberia were made by India’s Synercare Mumbai, according to the Nigerian regulator. The Liberian health regulator said it planned to incinerate the stock and also recall two other Synercare products as a precaution.
Synercare did not respond to a request for comment.
Not recommended
Since 2001, the WHO has recommended against giving cough syrups to children aged under 5, because it says there is limited evidence of how effective they are or what side effects they might have.
There have also been at least five incidents in the last half-century, when paracetamol and cough medicines were contaminated with deadly chemicals in countries including India and Panama, although the spate of deaths last year is the deadliest on record.
The WHO also urged all countries to step up surveillance and offered support to concerned countries that did not have the resources to test their own medicines.
“It’s not over, certainly,” said Kuwana. “But we don’t need to panic, as a lot of countries are now being proactive.”