Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
View all search resultsGovt arranging logistics, human resources for vaccination program: Task force
Change text size
Gift Premium Articles
to Anyone
T
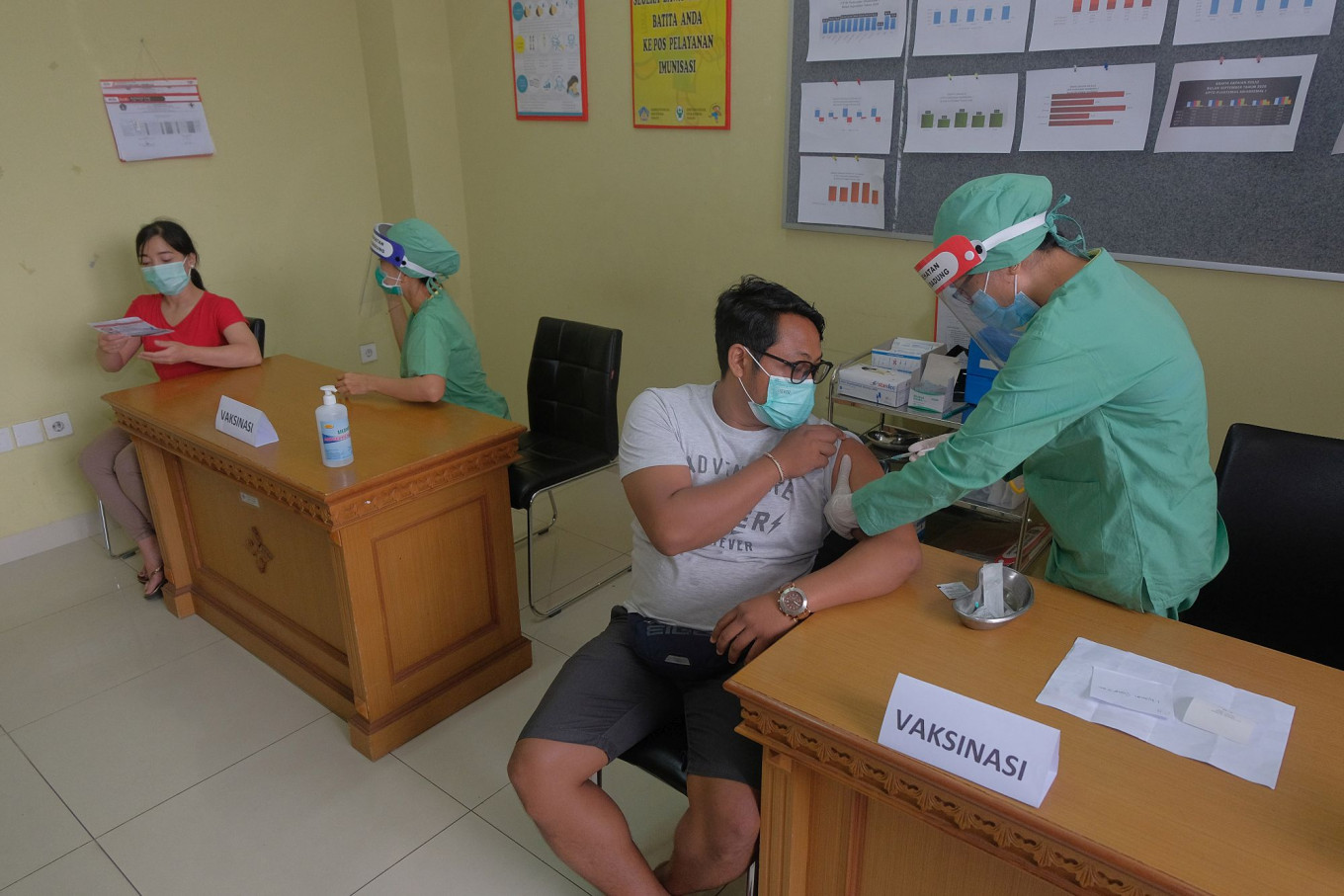
he Indonesian government has prepared logistics and human resources for the first round of the national COVID-19 vaccination program, which is seeks to begin in November or December.
Essential logistics for the vaccination drive include the so-called cold chain, a series of precisely coordinated events in a temperature-controlled environment to store, manage and transport vaccines in order to deliver them to all parts of the world.
According to the national COVID-19 task force, the Health Ministry has been working well in setting up the cold chain.
“The preparation [for] the cold chain in Indonesia has reached 97 percent,” task force spokesman Wiku Adisasmito said in a statement on Thursday.
Apart from the cold chain preparation, the government has also groomed 739,722 medical professionals as well as 23,145 vaccinators, especially to be assigned to community health centers (Puskesmas) and hospitals nationwide.
“We believe that a successful vaccination program is one that is medically safe and effective, and is thoroughly prepared. We hope the public are patient while waiting for the vaccination to roll out. In the meantime, please continue to wear your mask, wash your hands and exercise physical distancing,” said Wiku.
The spokesman went on to say that the government was still waiting for results from late-stage trials of would-be COVID-19 vaccines to ascertain their safety, detect possible side effects and decide on the proper dosage.
“The government is still waiting for the phase three [vaccine trial] results, which then need to be transferred to the Indonesian Food and Drug Monitoring Agency [BPOM] for further analysis,” added Wiku.
The task force has urged regional administrations to increase their testing efforts and has implored to people to have themselves checked up at health facilities as soon as they feel any COVID-19 symptoms.
Last week, President Joko “Jokowi” Widodo instructed his Cabinet to ensure the safety and effectiveness of the COVID-19 vaccines to be used in the national vaccination program.
The two issues were the top concerns of experts and the public, he said.
“Safety means that all vaccines given to people must first go through a series of proper clinical trials,” the President said during a limited Cabinet meeting at Merdeka Palace on Oct. 26. “Because if not – if there’s even one problem – it will create public distrust in the vaccination effort.”
State-owned pharmaceutical holding company PT Bio Farma, in partnership with China’s Sinovac Biotech Ltd., is conducting phase three clinical trials on a candidate vaccine in West Java. The trials are expected to end in January.
The country has also secured millions of doses of potential COVID-19 vaccines produced by Chinese firms Cansino and Sinopharm, Emirati artificial intelligence Group 42 (G42) Health Care and British pharmaceutical company AstraZeneca. (nkn)