Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
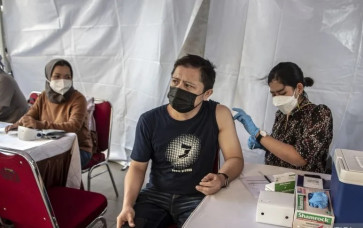
View all search resultsUncertainty looms over plan to use local vaccines as booster shots
It remains unclear whether the public will be able to use the two locally developed vaccines for the much-needed booster doses with the government slashing the national economic recovery (PEN) budget for the pandemic response next year and possibly cutting funding for further research.
Change text size
Gift Premium Articles
to Anyone
Indonesia has almost finished the last round of clinical trials for the two long-awaited locally produced COVID-19 vaccines.
But the future remains unclear whether the public could use them for much-needed booster doses with the government slashing the national economic recovery (PEN) budget for the pandemic response next year and possibly cutting the funding for further research.
A research team from Airlangga University in East Java, in cooperation with local firm Biotis Pharmaceuticals Indonesia, has recently finished injecting some 4,000 volunteers aged 18 and above with the second dose of the homegrown vaccine candidate, as part of the third stage of clinical trials. The vaccine is named Merah Putih – after the color of Indonesia's red-and-white flag.
The team said so far, volunteers had not experienced severe adverse effects from the vaccine, which was developed using inactivated coronavirus.
The team is expecting to apply for Emergency Use Authorization (EUA) for the vaccine as primary doses with the Food and Drug Monitoring Agency (BPOM) sometime next month after they collect immunogenicity data from the volunteers.
"In around four weeks we will conduct blood tests on the volunteers to see the vaccine immunogenicity. We can request an EUA from the BPOM once the immunogenicity data is available," researcher Dominicus Husada told The Jakarta Post on Wednesday. "The manufacturing process for the vaccine can start as soon as the BPOM grants us the EUA."
Without revealing a specific timeline, professor Ni Nyoman Tri Puspaningsih, coordinator for COVID-19 products research at Airlangga University, said that Biotis was capable of producing around 20 million doses of the Merah Putih vaccine.