Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
View all search resultsUS approves first one-dose coronavirus vaccine
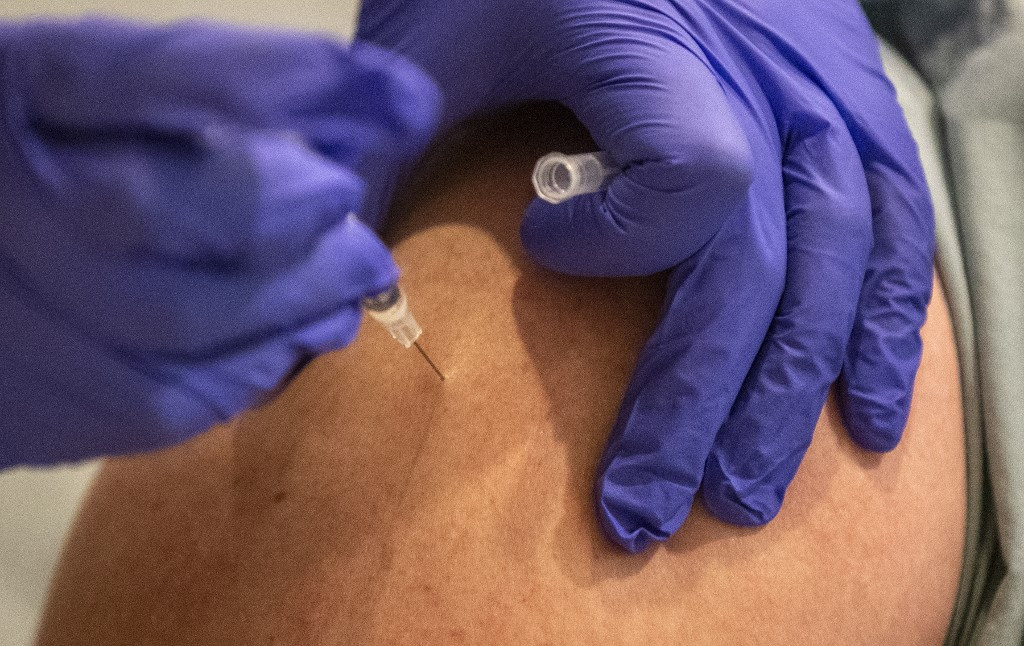
The vaccine is raising hope for a simpler mass inoculation process, given that the current two approved vaccines require two shots. It can be stored for three months at normal refrigerator temperatures and has a two-year shelf-life when frozen.
Change text size
Gift Premium Articles
to Anyone
T
he US drug regulator on Saturday granted emergency use authorization for Johnson & Johnson's coronavirus vaccine, the third such vaccine it has approved and the first to offer protection with just one shot.
The vaccine is raising hope for a simpler mass inoculation process, given that the current two approved vaccines require two shots. It can be stored for three months at normal refrigerator temperatures and has a two-year shelf-life when frozen.
The US Food and Drug Administration has confirmed the safety of the vaccine based on data provided by the major US pharmaceutical company from a late-stage clinical trial.
The shot is 66 percent effective overall in preventing moderate to severe COVID-19 cases 28 days after vaccination, according to the data. For severe disease alone, it was about 85 percent effective.
The study results have included efficacy against newly emerging strains of coronavirus, including some highly infectious variants present in the United States, Latin America and South Africa, according to the company.
The vaccine has been approved for use in people 18 years of age and older.
In the United States, two types of two-dose vaccines were granted emergency use authorization in December -- one developed by US pharmaceutical giant Pfizer Inc. and its German partner BioNTech, and the other from Moderna.
To create antibodies, both use messenger RNA, or mRNA, which is genetic material, to give instructions for cells to make a harmless "spike protein" that resembles one found in the novel coronavirus.
The Johnson & Johnson vaccine instead uses a modified version of a common virus to deliver DNA into cells to make such a spike protein.