Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
View all search resultsChinese coronavirus vaccine could be tested, manufactured in Canada
Change text size
Gift Premium Articles
to Anyone
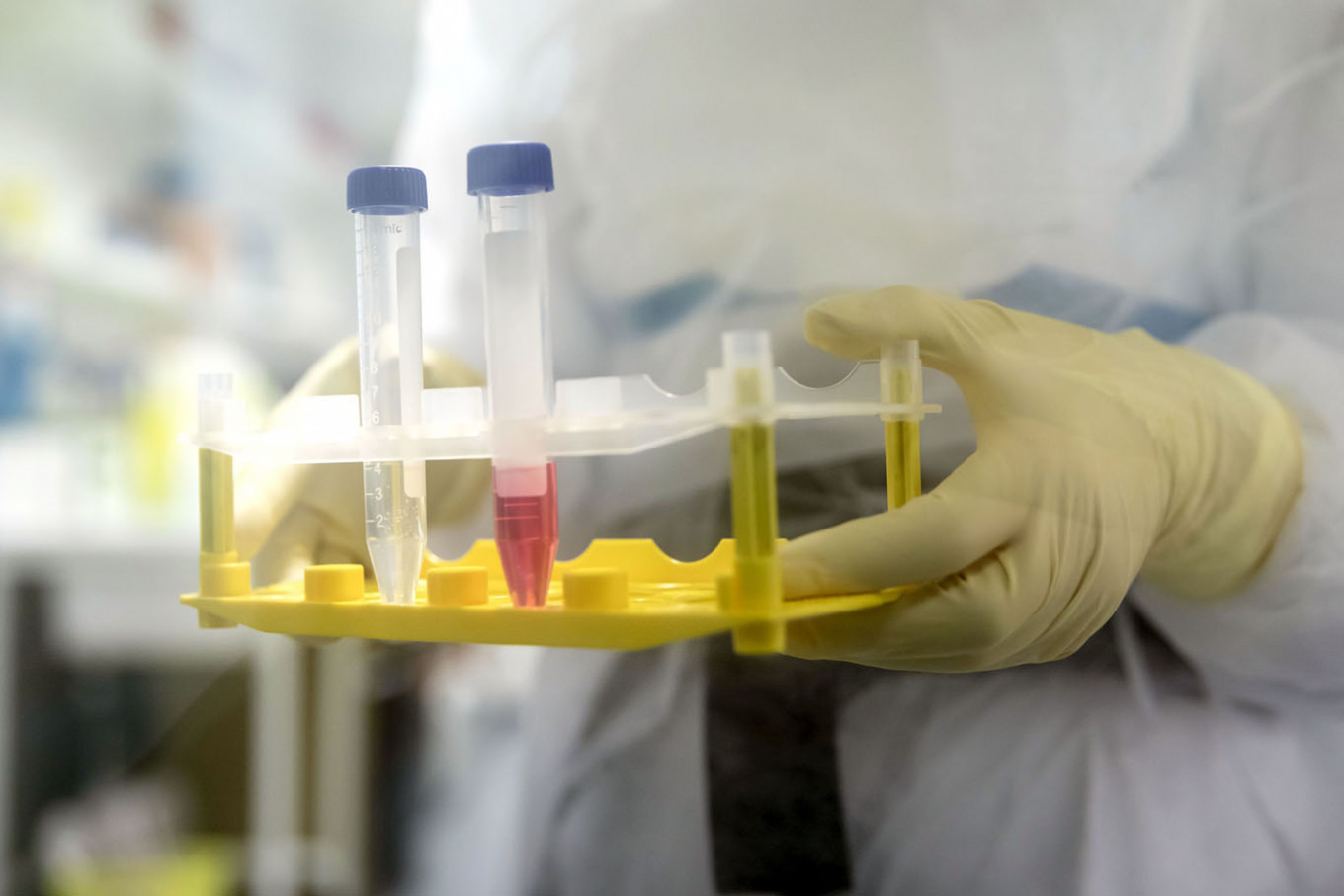 A French National Centre for Scientific Research (CNRS) researcher holds up a test tube containing cells to be infected with COVID-19 during coronavirus vaccine research work inside the Pasteur Institute laboratories in Lille, France, on Monday, March 9, 2020. China's CanSino Biologics Inc , the company behind one of the few coronavirus vaccine candidates already in clinical trials, is collaborating with Canada's National Research Council to "pave the way" for future trials in Canada, the research council said on Tuesday.
(Bloomberg/Adrienne Surprenant)
A French National Centre for Scientific Research (CNRS) researcher holds up a test tube containing cells to be infected with COVID-19 during coronavirus vaccine research work inside the Pasteur Institute laboratories in Lille, France, on Monday, March 9, 2020. China's CanSino Biologics Inc , the company behind one of the few coronavirus vaccine candidates already in clinical trials, is collaborating with Canada's National Research Council to "pave the way" for future trials in Canada, the research council said on Tuesday.
(Bloomberg/Adrienne Surprenant)
C
hina's CanSino Biologics Inc , the company behind one of the few coronavirus vaccine candidates already in clinical trials, is collaborating with Canada's National Research Council to "pave the way" for future trials in Canada, the research council said on Tuesday.
The NRC said it would scale up a production process for CanSino's vaccine candidate at a government facility in Montreal, and that CanSino was preparing a clinical trial application for Health Canada, the country's drug regulator.
The CanSino vaccine is in early trials, and there is no way to know whether it will work. But if it does, the collaboration could help ensure that Canadians have access to it. Local trial data could reassure Health Canada that the vaccine is safe, and local manufacturing could ensure some doses are at hand.
A vaccine that protects people from the coronavirus could eventually end the pandemic, but finding one that works and manufacturing enough doses is a huge challenge.
CanSino's vaccine is produced using a cell line that was developed at the NRC, the agency said, and the two organizations have worked together since 2013. The company used the same cell line to develop an Ebola vaccine.
NRC and Health Canada did not immediately respond to questions about whether the collaboration would make it possible for Health Canada to consider trial data gathered in China in eventually evaluating the vaccine.
Shares of Tianjin-based, Hong Kong-listed CanSino rose on April 26 after the company said Health Canada had agreed to meet to discuss a clinical trial application.









