Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
View all search resultsS. Korea's Daewoong Pharma gets India nod to test anti-parasite drug for COVID-19
Change text size
Gift Premium Articles
to Anyone
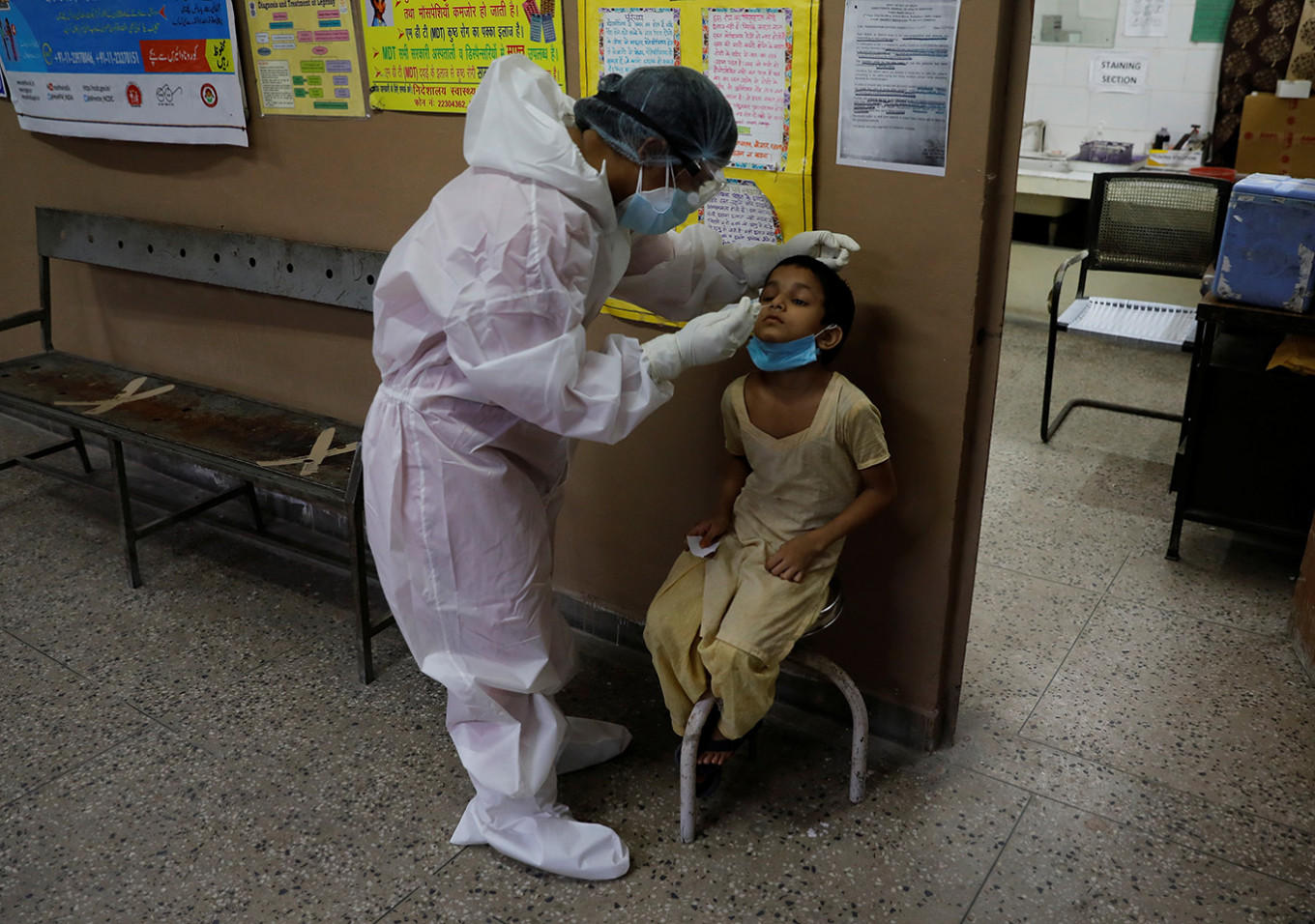 A health worker in personal protective equipment (PPE) collects a sample using a swab from a person at a local health centre to conduct tests for the COVID-19 in New Delhi, India August 7, 2020.South Korea's Daewoong Pharmaceutical Co Ltd said on Tuesday it received Indian regulatory approval to test its anti-parasitic niclosamide drug to treat COVID-19 patients in an early-stage human trial. (REUTERS/Adnan Abidi)
A health worker in personal protective equipment (PPE) collects a sample using a swab from a person at a local health centre to conduct tests for the COVID-19 in New Delhi, India August 7, 2020.South Korea's Daewoong Pharmaceutical Co Ltd said on Tuesday it received Indian regulatory approval to test its anti-parasitic niclosamide drug to treat COVID-19 patients in an early-stage human trial. (REUTERS/Adnan Abidi)
S
outh Korea's Daewoong Pharmaceutical Co Ltd said on Tuesday it received Indian regulatory approval to test its anti-parasitic niclosamide drug to treat COVID-19 patients in an early-stage human trial.
The phase 1 trial, approved by India's Central Drugs Standard Control Organization (CDSCO), will involve around 30 healthy participants to test safety and kickstart this month, Daewoong said in a statement.
The South Korean drugmaker is testing the drug in partnership with New Delhi-based Mankind Pharma Ltd, which will continue the second and third phases of trials in India on mild and severe coronavirus patients.
Drugmakers worldwide are scrambling to develop treatments for the illness caused by the novel coronavirus, which has killed nearly 739,000 people globally since it first emerged late last year in China.
The trial results from India will be used for export permits in Europe and the United States, said Daewoong. The company is also waiting for a separate phase 1 trial approval from South Korea, Nathan Kim, vice-president at Daewoong's communications office, told Reuters.
Daewoong had said its anti-viral drug had completely eliminated the novel coronavirus from animals' lungs during pre-clinical testing.









