Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
View all search resultsIndonesia can be manufacturing hub for COVID-19 vaccine, says Chinese foreign minister
The Chinese Center for Disease Control and Prevention (CDC) said COVID-19 vaccines being developed in China may be ready for use by the general public as early as November.
Change text size
Gift Premium Articles
to Anyone
C
hina stepped up its efforts in "vaccine democracy” over the weekend, with Chinese Foreign Minister Wang Yi saying that joint vaccine programs between Indonesian and China could be the focus of new ties between the two countries.
Speaking after a meeting with visiting Coordinating Maritime Affairs and Investment Minister Luhut Pandjaitan, Wang said Indonesia could be a vaccine production hub for Southeast Asia in the near future.
"China is willing to work with Indonesia on vaccine research, production and distribution, and support exchanges of relevant departments and medical institutes to help ensure access to affordable vaccines across the region and around the world," Wang said.
The Chinese Center for Disease Control and Prevention (CDC) said COVID-19 vaccines being developed in China may be ready for use by the general public as early as November.
China has four COVID-19 vaccines in the final stage of clinical trials. At least three of those have already been offered to essential workers under an emergency use program launched in July. Phase 3 clinical trials are proceeding smoothly and the vaccines could be ready for the public in November or December.
Beijing has promised that Southeast Asian countries would be among the first to receive the COVID-19 vaccines it develops once they become available.
Responding to Wang's statement, Luhut said he expected that following the Chinese government's commitment, many Indonesian doctors and health workers could join an exchange program and learned from the best practices in China.
"I want more cooperation from our hospitals, our doctors and health workers as well as research and technological cooperation between the two countries," Luhut said in a statement made available to The Jakarta Post.
Read also: Jokowi issues presidential regulation on COVID-19 vaccination
Luhut was traveling with Health Minister Terawan Agus Putranto and director of state-run pharmaceutical company PT Bio Farma Honesti Basyir to finalize the procurement of a COVID-19 vaccine to be used by the government in a mass vaccination program beginning in November.
Luhut and his team held a meeting with three pharmaceutical firms: Sinovac, Sinopharm and CanSino Bio.
Vaccines produced by the three companies have entered Phase 3 clinical trials and are in the process of getting emergency use authorization in some countries.
Beijing has promised that Southeast Asian countries would be among the first to receive the COVID-19 vaccines it develops once they become available. (JP/-)In recent months, CanSino has undertaken Phase 3 clinical trials in China, Saudi Arabia, Russia and Pakistan. Sinophram, meanwhile, has carried out clinical trials in China, the United Arab Emirates, Peru, Morocco and Argentina. Sinovac has also carried out Phase 3 clinical trials in China, Indonesia, Brazil, Turkey, Bangladesh and Chile.
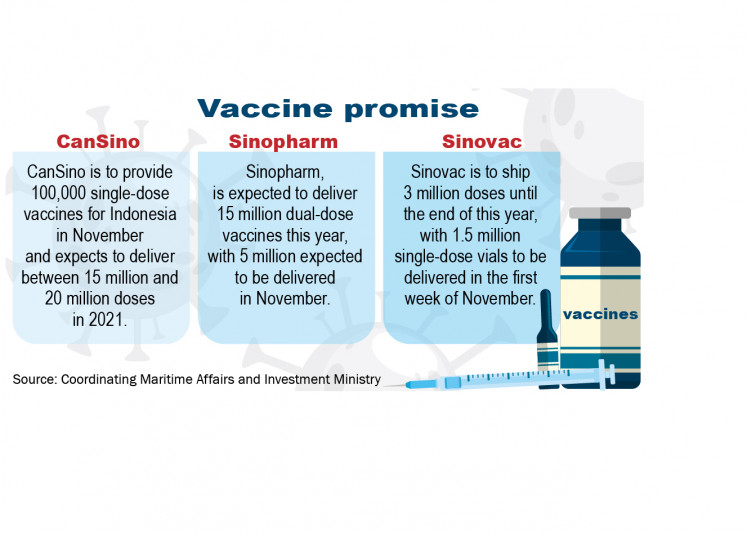
CanSino has agreed to provide 100,000 single-dose vaccines for Indonesia in November and expects to deliver between 15 million and 20 million doses in 2021. Sinopharm, meanwhile, is expected to deliver 15 million dual-dose vaccines this year, with 5 million expected to be delivered in November. Sinovac has pledged to ship 3 million doses until the end of this year, with 1.5 million single-dose vials to be delivered in the first week of November.
President Joko “Jokowi” Widodo has said that the country would have the ability to procure around 290 million doses of a COVID-19 vaccine by the end of 2021.
Other than relying on foreign vaccine manufacturers, Indonesia is also developing its own vaccine named Merah Putih, in reference to the country’s red and white flag.
The Merah Putih vaccine, which is being developed by a national consortium under the Research and Technology Ministry, is expected to cover at least 50 percent of the country’s 260 million people. State-owned pharmaceutical company Bio Farma aims to mass-produce the vaccine by 2022 if it gets approval from the Indonesia Food and Monitoring Agency (BPOM).
Editor’s note: This article is part of a public campaign by the COVID-19 task force to raise people’s awareness about the pandemic.