Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
View all search resultsNo emergency vaccine approval this year in Indonesia, says BPOM
“We don’t have the finished goods yet, so we can’t just make guesses,” the health minister said.
Change text size
Gift Premium Articles
to Anyone
I
ndonesia may not be able to start its proposed mass vaccination program this year because of efficacy concerns, despite President Joko “Jokowi” Widodo’s push to fast-track the effort amid rising cases and rampant health protocol violations.
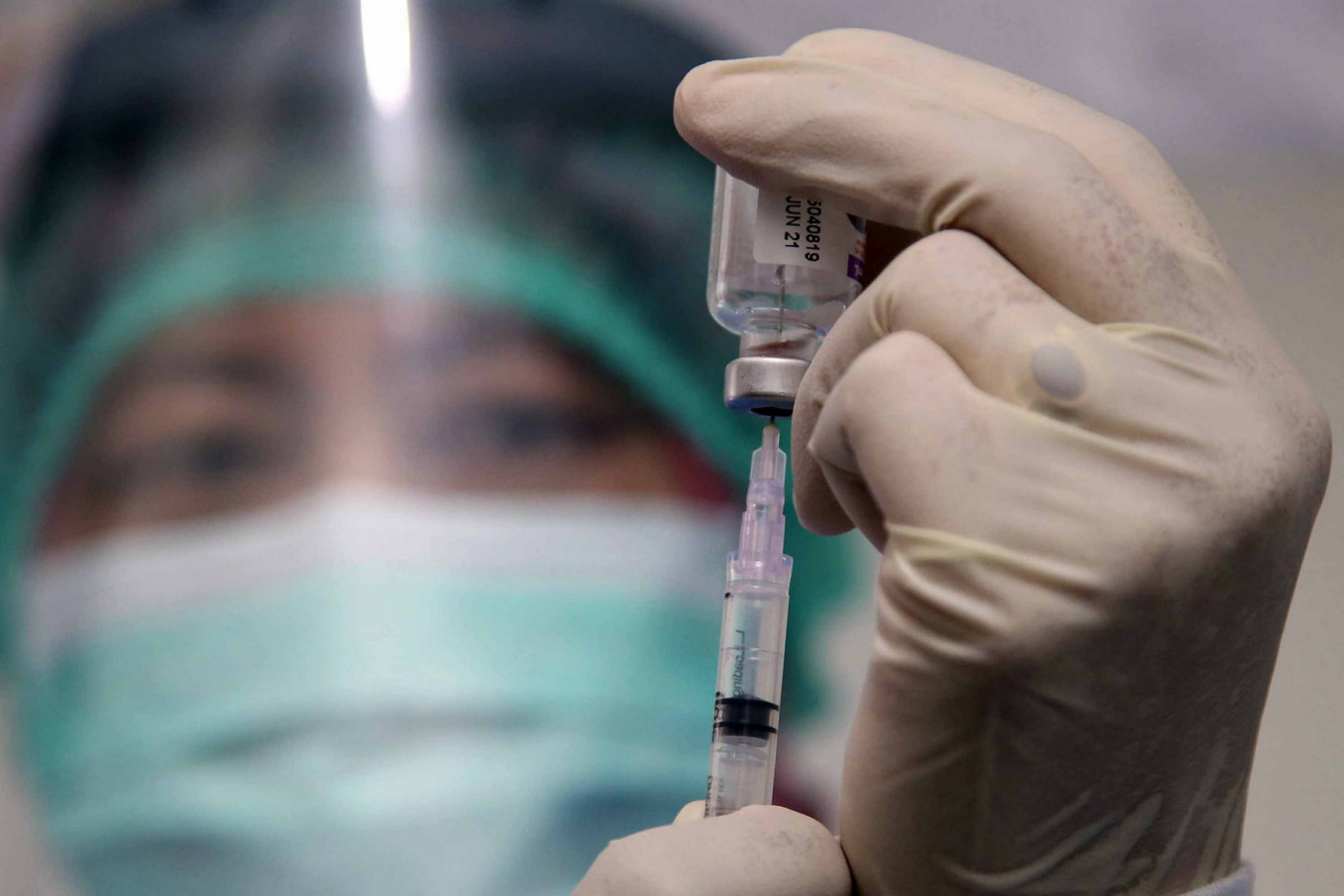
The Indonesian Food and Drug Monitoring Agency (BPOM) has said it will not authorize the emergency use of a COVID-19 candidate vaccine in December because of a lack of data on its effectiveness.
However, the agency has offered an alternative restricted vaccination program through a “compassionate use” provision, where a potential vaccine whose efficacy has not been proven may be used in a limited capacity.
BPOM head Penny Kusumastuti Lukito told lawmakers on Tuesday that during an inspection of Sinovac Biotech facilities in China, her agency had obtained data from the first two phases of the candidate vaccine’s clinical trials. The data from the final stage of testing, taking place in Indonesia, would be available in late November.
The Sinovac candidate vaccine is one of several potential COVID-19 vaccines in final-stage testing globally. Sinovac has said it is confident about the safety of the potential vaccine.
However, according to Penny, final data on the candidate vaccine’s efficacy will arrive later than expected. This information is required before the vaccine’s approval for emergency use, as agreed in a World Health Organization forum on vaccine consultation on Nov. 6.
The forum was attended by world drug regulators, including the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA).