Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
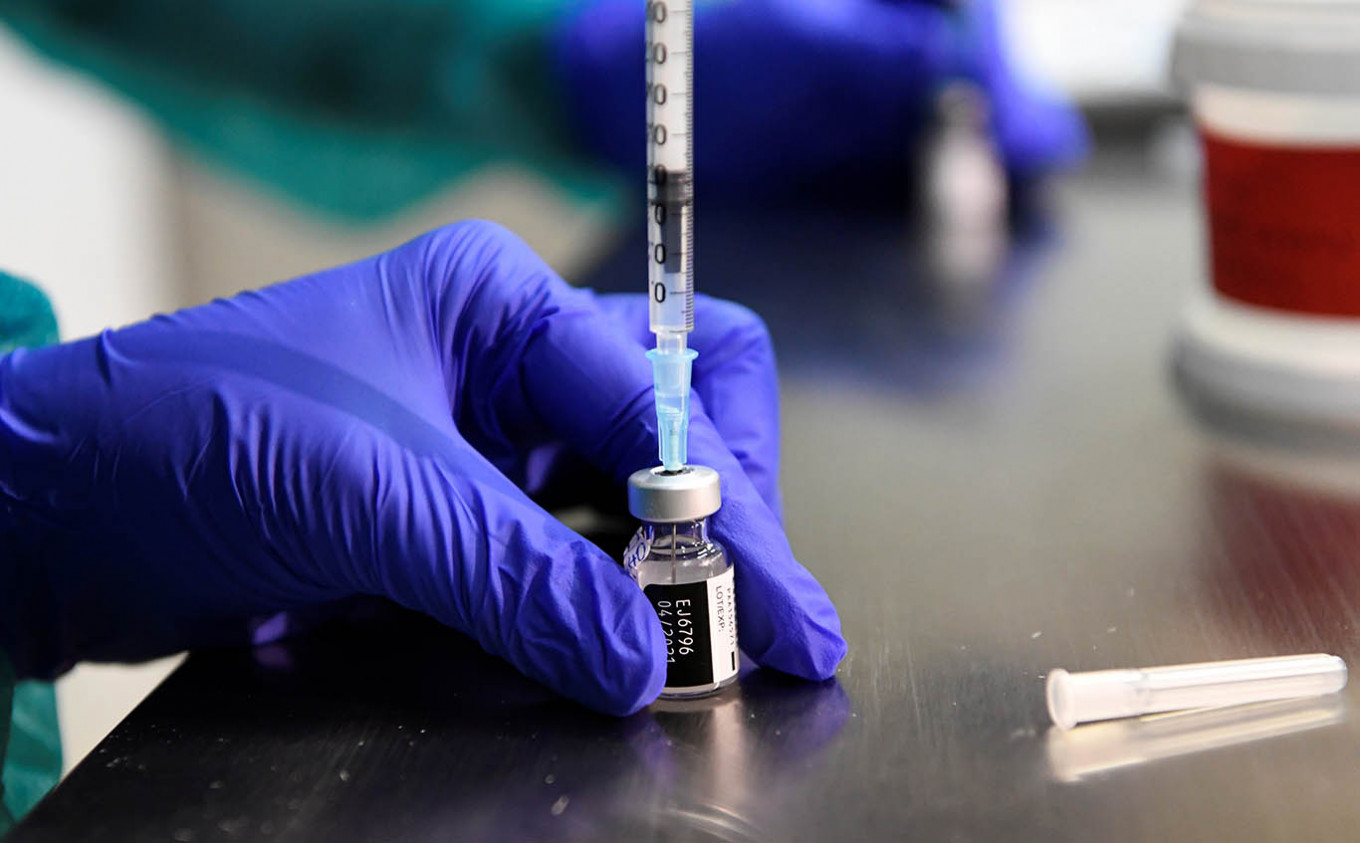
View all search resultsIndonesia to strike vaccine deals with Pfizer, AstraZeneca
The government has diversified its forthcoming COVID-19 vaccine supplies to account for setbacks, Health Minister Budi Gunadi Sadikin has said.
Change text size
Gift Premium Articles
to Anyone
N
ewly appointed Health Minister Budi Gunadi Sadikin has said the government is currently finalizing deals with United States vaccine producer Pfizer and United Kingdom-based AstraZeneca to secure enough stockpile to carry out its long-awaited vaccination drive next year.
Once the procurement deals are signed, the former banker said, Indonesia would finally be able to reach the minimum number of vaccine doses required to be able to achieve herd immunity, whereby large sections of the population develop immunity to stop the virus in its tracks.
The announcement was Budi’s first big break since being appointed last week to replace former minister Terawan Agus Putranto, who had led a poor pandemic response that saw the nation still struggle to contain COVID-19.
In the nine months since it first reported cases of the virus, Indonesia has recorded over 727,000 confirmed infections and more than 21,000 deaths, according to the latest official tally by the national COVID-19 task force.
Speaking to the media on Tuesday, Budi said that at least 181 million people out of the nation’s 269 million-strong population would need to be inoculated in order to reach the desired coverage to trigger herd immunity.
Assuming each person requires two jabs of the vaccine, and that 15 percent in wastage needs to be accounted for as recommended by the World Health Organization, he said Indonesia needed to secure a total of 426 million doses of COVID-19 vaccines.
So far, the government has secured 125 million doses from Chinese firm Sinovac and another 130 million from US-based Novavax, with more optional supplies on offer.
Critics have raised concerns that Indonesia is pinning too much hope on Sinovac’s CoronaVac vaccine, which had its first shipment arrive in the country early this month. Unlike other potential vaccines that have shown promising efficacy results from late stage trials, conclusive data on Sinovac’s product was not yet available.
BioNTech-Pfizer and AstraZeneca have reported over 90 percent and 70 percent efficacy for their candidate vaccines, respectively.
Meanwhile, Novavax has only begun with its large late stage clinical trial of its vaccine in the US and Mexico, Reuters reported.
Read also: How CoronaVac's efficacy, new virus strains will affect Indonesia's vaccination blitz
Budi said the government would soon be signing a contract with AstraZeneca for a total of 100 million doses of vaccine, although only half of that are firm orders. A similar arrangement is also expected with BioNTech-Pfizer for 100 million doses of its vaccine, split into 50 million doses of firm orders and the rest as optional supplies.
“We hope we will be able to finalize the deals with AstraZeneca and Pfizer in the near future,” he said.
According to presentation materials by the Health Ministry, the AstraZeneca deal will likely be inked in the coming days, while the Pfizer deal is expected early next year.
The government had worked around the fact that some of its vaccine supplies could be secured from the COVAX Facility, a multilateral initiative led by the WHO, the GAVI vaccine alliance and the Coalition for Epidemic Preparedness Innovations (CEPI). The initiative aims to fairly distribute 2 billion vaccine doses by the end of 2021.
Budi said the number of doses Indonesia may get from COVAX was still not locked in; it could either receive enough doses for 3 percent of the population, equal to 16 million doses of vaccine, or for 20 percent of the population (around 100 million doses).
“That is why we have prepared contracts with options from the four vaccine suppliers. If a decision is made on GAVI’s vaccine – which is free – we won’t have to buy the options, but if it won’t be delivered according to our desired schedule, Indonesia still would have secured enough supplies from the other companies,” he explained.
According to the government’s plans, the vaccination drive is expected to begin in earnest in mid-January, or after the Sinovac vaccine gets emergency use authorization by the Food and Drug Monitoring Agency (BPOM).
It will begin with the vaccination of frontline medical practitioners all around the country, before moving on to different sections of the population.
Like any other country bogged down by the COVID-19 outbreak, Indonesia has also been competing in the race to secure access to various candidate vaccines.
Budi dismissed concerns that the government was too dependent on the Chinese vaccine, saying the nation had diversified its vaccine supplies. He said Indonesia was confident it had sufficient supplies on hand even if some of the vaccines ended up failing the trials or deliveries got delayed.
“Out of around 400 million doses in total, around 100 million vaccines will be imported from China; around 100 million will be imported from Novavax, which is an American-Canadian company; 100 million will be imported from AstraZeneca, which is based in London, England; then 100 million of the Pfizer vaccine, which is a joint venture between Germany and the US,” he said.