Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
View all search resultsLocal vaccine efforts face old problems
Change text size
Gift Premium Articles
to Anyone
I
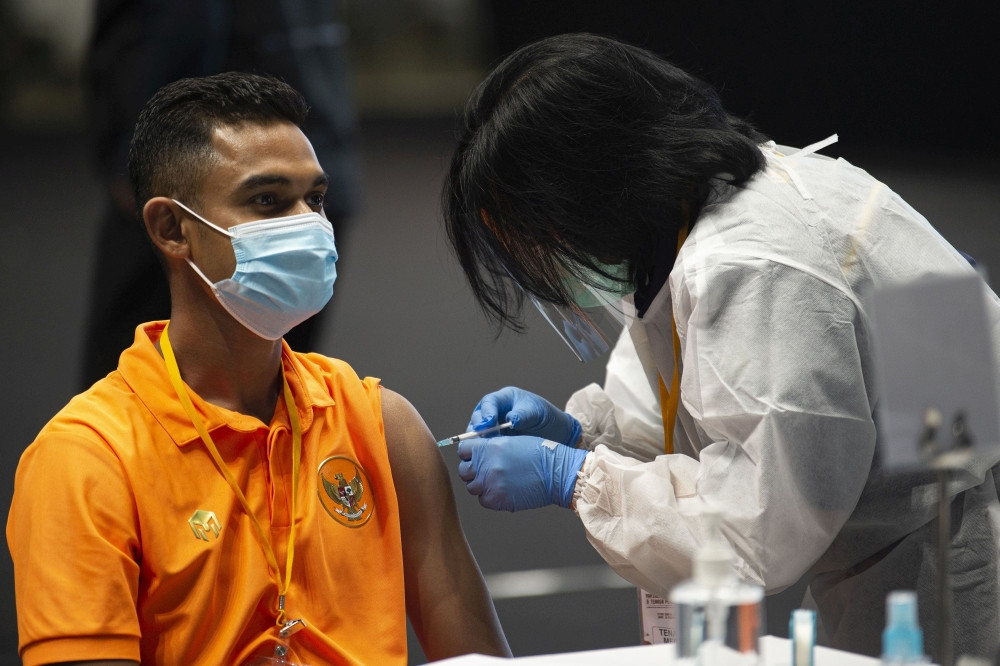
ndonesian scientists developing vaccines are hoping to defend the country against the onslaught of COVID-19 but they still must deal with the same old issues that have long hampered research in the country, from overreliance on imported materials to lack of experience in downstreaming research products.
The government has long been aware that Indonesia, the world’s fourth-largest country by population, cannot solely rely on vaccine diplomacy to reach the desired inoculation coverage amid growing protectionism and limited supplies across the globe caused by restrictions during the pandemic.
Shortly after Indonesia reported its first confirmed COVID-19 cases last year, several scientists from a variety of institutions began to look into how they could develop vaccines against the coronavirus. Months later, a consortium helmed by the Research and Technology Ministry-National Research Agency (BRIN) was set up in two research institutions and four universities, each working on their own experimental Merah Putih (Red and White) vaccine using various platforms.
Research Minister Bambang Brodjonegoro said in December 2020 that Rp 300 billion (US$20.6 million) would go into the Merah Putih vaccine development this year, and Health Minister Budi Gunadi Sadikin said recently that his office was allocating Rp 400 billion for these efforts. But the issues faced by domestic vaccine research and development run deeper than funding, which in fact also lags far behind those of neighboring countries relative to their GDPs.
The Jakarta Post talked to five researchers involved in the Merah Putih vaccine development, almost all of whom pointed to Indonesia’s underdeveloped upstream chemical industry as the “classic problem" that leads to overreliance on imported materials.
They said importing materials into Indonesia to develop a new vaccine was an overly time-consuming, costly process that might take more than two months. The bureaucracy abroad and at home, including customs, does not help -- neither are global border restrictions observed throughout the COVID-19 pandemic.
“If these issues are not solved, I think efforts to speed up innovation will continue to face problems,” said biotechnology researcher Wien Kusharyoto from the Indonesian Institutes of Sciences (LIPI), which is part of the consortium developing the Merah Putih vaccine.
The vaccine development by Wien's team is an example of how their efforts have been hampered by the late arrival of chemicals needed for vaccine research. His team was still halfway through the process of developing the seed vaccines, he said.
Reliance on imports, however, is not limited to necessary chemicals, but also lab equipment and experimental animals for preclinical trials. The COVID-19 pandemic has presented the country with its first ever experience to develop vaccines starting from scratch.
Preclinical trials on the University of Airlangga’s (UNAIR) experimental inactivated virus Merah Putih vaccine could have started in February, if not for this problem, said Ni Nyoman Tri Puspaningsih who leads the university's Research Center for Biomolecular Engineering.
The trials have only started this month, carried out by its partnering firm Biotis Pharmaceuticals. Ni Nyoman said the research ministry has helped accelerate the process, otherwise it could have taken much longer.
Read also: Slow progress, questionable research in Indonesian vaccine development efforts
A fresh plan regarding the research ministry’s possible split with BRIN, which will see the former merge with the Education and Culture Ministry consequently poses questions as to whether the education ministry will be able to, for the first time, shoulder the task of speeding up innovation and vaccine development.
“The merger of the research ministry could potentially disrupt Merah Putih vaccine development […] the development must be fully supported rather than being disrupted by these events,” said Netty Prasetiyani, member of House of Representatives Commission IX overseeing health.
Researchers said in general they were not concerned about future funding for vaccine development, which is currently overseen by BRIN, given that after lab work is complete, the costs of clinical trials and production are to be borne by the Health Ministry, the State-Owned Enterprise Ministry and pharmaceutical industry.
They recommend that the consortium be maintained to ensure that universities, which are under the supervision of the education ministry, and research institutions that are currently under BRIN can still work closely together even after the major restructure.
“It’ll be tougher on the Education and Culture Ministry, given the COVID-19 pandemic. [...] There must not be any setbacks caused by its focus on handling the education,” UNAIR’s Ni Nyoman said.
Wiku Adisasmito, the government’s spokesperson on COVID-19 affairs who also heads the COVID-19 research consortium, said the government had undertaken thorough deliberation regarding the BRIN-research ministry split. He said with or without the split, the government would continue its commitment toward COVID-19 vaccine development to meet domestic and global needs.
But for Amin Soebandrio, the head of the Eijkman Institute for Molecular Biology, it is how the country evaluates research that also needs fixing. “It’s seen in the same way as building a house. If the funding is disbursed, then it’s expected that everything will fall into schedule with the result to be predicted from the beginning. With research, results can’t be [predicted] and different results don’t mean they have failed,” he said.
Eijkman is partnering with state-owned firm Bio Farma, expecting to roll out its Merah Putih clinical trials by the fourth quarter of 2021 and securing emergency use authorization in mid-2022 before it can be mass-produced should things go as planned.
Read also: Indonesian government promises big, then falters in vaccine procurement
Public accountability is also the reason why the University of Indonesia team expects to secure funding from local private investors first, especially as it is using a relatively uncommon vaccine platform of DNA, its microbiologist Budiman Bela said. Should things run smoothly, only then will the university expect more funding assistance from the government, he said. His team is currently in talks with a local firm for partnership in running preclinical trials.
Of the six institutions involved in vaccine development, only UNAIR and Eijkman have partnering firms -- but even then some technology adjustment will be necessary for them. Research Minister Bambang has acknowledged the need to upscale the country’s pharmaceutical industry for the Merah Putih vaccine development.
The country’s most experienced firm in vaccine production, Bio Farma, for now has only the capacity to produce inactivated virus vaccines and recombinant protein-based vaccines. The firm's president director Honesti Basyir said it was preparing facilities to produce protein-based vaccines, which are rather new for the firm, and that it was considering working with private firms.
“Not many firms in Indonesia are ready to develop vaccines. The issue of different technology platforms used also presents an obstacle to upscaling from research to production,” said clinical microbiologist Tri Wibawa from Gadjah Mada University, who expects to develop seed vaccines by the end of 2021.