Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
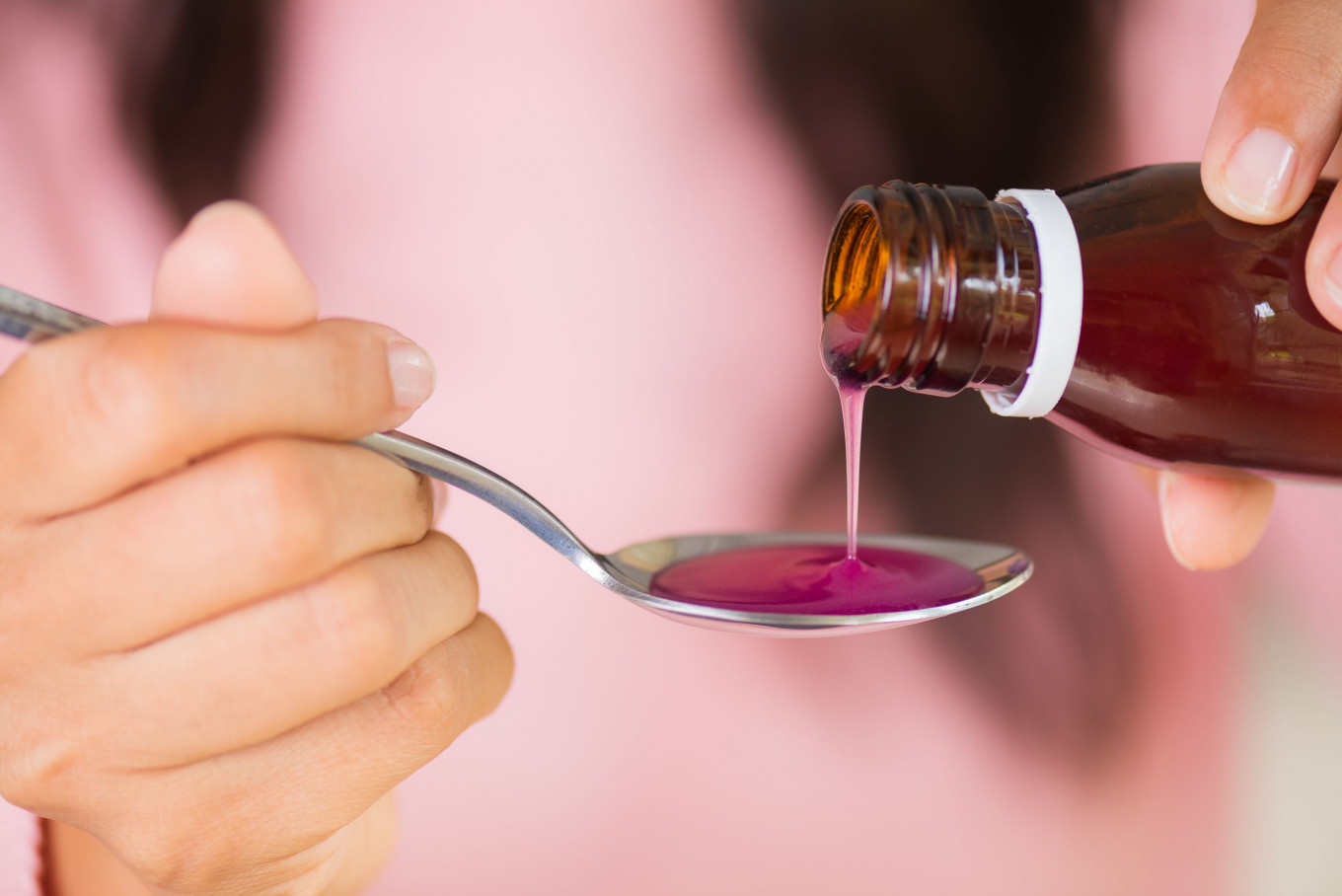
View all search resultsWHO says more contaminated medicinal syrups found in new regions
The alert is the latest in a line of warnings from WHO about similarly contaminated medicines made in India and Indonesia, which were linked to the deaths of around 300 children worldwide last year.
Change text size
Gift Premium Articles
to Anyone
The World Health Organization (WHO) on Thursday said several contaminated syrups and suspension medicines had been identified in countries in the regions of the Americas, the Eastern Mediterranean, Southeast Asia and the Western Pacific.
The affected products were manufactured by Pharmix Laboratories in Pakistan, the WHO said, and were first identified in the Maldives and Pakistan. Some of the tainted products have also been found in Belize, Fiji and Laos. Pharmix was not immediately available for comment.
The medicines, liquids containing active ingredients to treat various conditions, contained unacceptable levels of the contaminant ethylene glycol, WHO said.
The alert is the latest in a line of warnings from WHO about similarly contaminated medicines made in India and Indonesia, which were linked to the deaths of around 300 children worldwide last year.
No adverse events have been reported to the WHO regarding the Pakistan-made syrups, the agency's statement said, but it urged countries to step up vigilance and test products made by the company between December 2021 and December 2022.
The contamination was found in Alergo syrup in a routine examination by the Maldives Food and Drug Authority in November, and confirmed by the Australian regulator.
A follow-up inspection at Pharmix manufacturing facilities, conducted by the Drug Regulatory Authority of Pakistan, found that a number of other products were also contaminated. It has ordered the company to stop making all oral liquid medicines and issued a recall alert in November.
A total of 23 batches of Alergo syrup, Emidone suspension, Mucorid syrup, Ulcofin suspension and Zincell syrup are affected, the WHO said. Only Alergo so far has been found outside Pakistan.
The contamination levels ranged from 0.62 to 0.82 percent, compared to the accepted level of not more than 0.10 percent, according to the alert. The products are variously designed to treat allergies, coughs and other health issues.
"The substandard products referenced in this alert are unsafe and their use, especially in children, may result in serious injury or death," the WHO warned.