Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
View all search resultsElon Musk's Neuralink says cleared for human test of brain implants
Neuralink said clearance from the US Food and Drug Administration (FDA) for its first in-human clinical study is "an important first step" for its technology, which is intended to let brains interface directly with computers.
Change text size
Gift Premium Articles
to Anyone
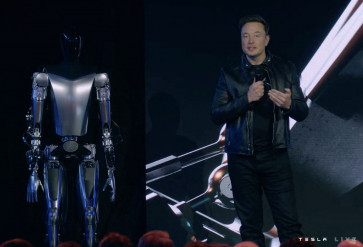
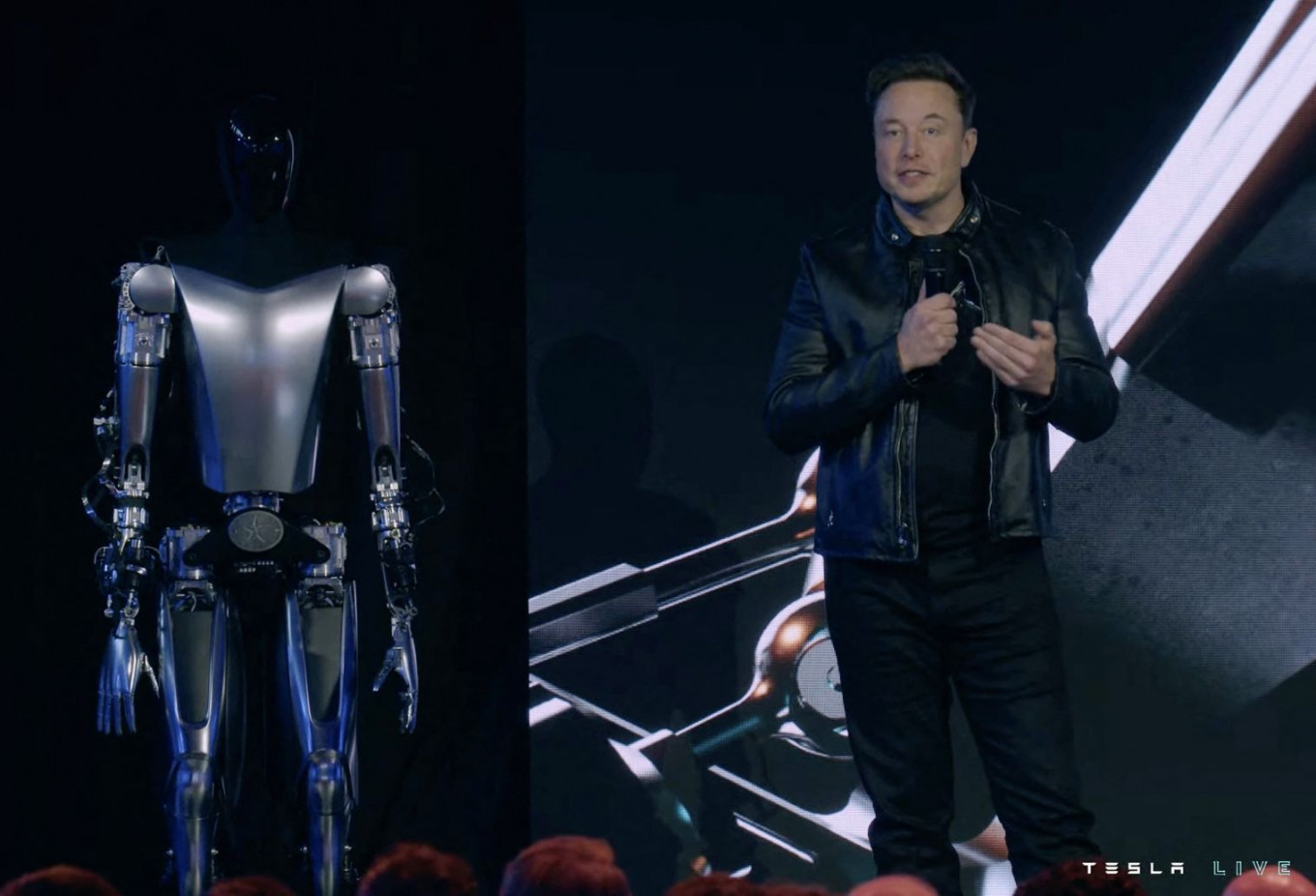 This video screen grab made from Tesla AI Day 2022 livestream shows Elon Musk standing on stage next to Optimus the humanoid robot in Palo Alto, California on September 30, 2022. Elon Musk presented on September 30, 2022 a prototype of the humanoid robot Optimus, which his company Tesla hopes to produce one day by “millions“ to “transform civilization“ and build a “future of abundance“ where poverty will be gone. (AFP/Tesla)
This video screen grab made from Tesla AI Day 2022 livestream shows Elon Musk standing on stage next to Optimus the humanoid robot in Palo Alto, California on September 30, 2022. Elon Musk presented on September 30, 2022 a prototype of the humanoid robot Optimus, which his company Tesla hopes to produce one day by “millions“ to “transform civilization“ and build a “future of abundance“ where poverty will be gone. (AFP/Tesla)
Elon Musk's start-up Neuralink on Thursday said it has approval from US regulators to test its brain implants in people.
Neuralink said clearance from the US Food and Drug Administration (FDA) for its first in-human clinical study is "an important first step" for its technology, which is intended to let brains interface directly with computers.
"We are excited to share that we have received the FDA's approval to launch our first-in-human clinical study," Neuralink said in a post on Musk-run Twitter.
Recruitment for a clinical trial is not yet open, according to Neuralink.
The aim of Neuralink implants is to enable human brains to communicate directly with computers, Musk said during a presentation by the start-up in December.
"We've been working hard to be ready for our first human (implant), and obviously we want to be extremely careful and certain that it will work well before putting a device in a human," he said at the time.
Neuralink prototypes, which are the size of a coin, have been implanted in the skulls of monkeys, demonstrations by the startup showed.