Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
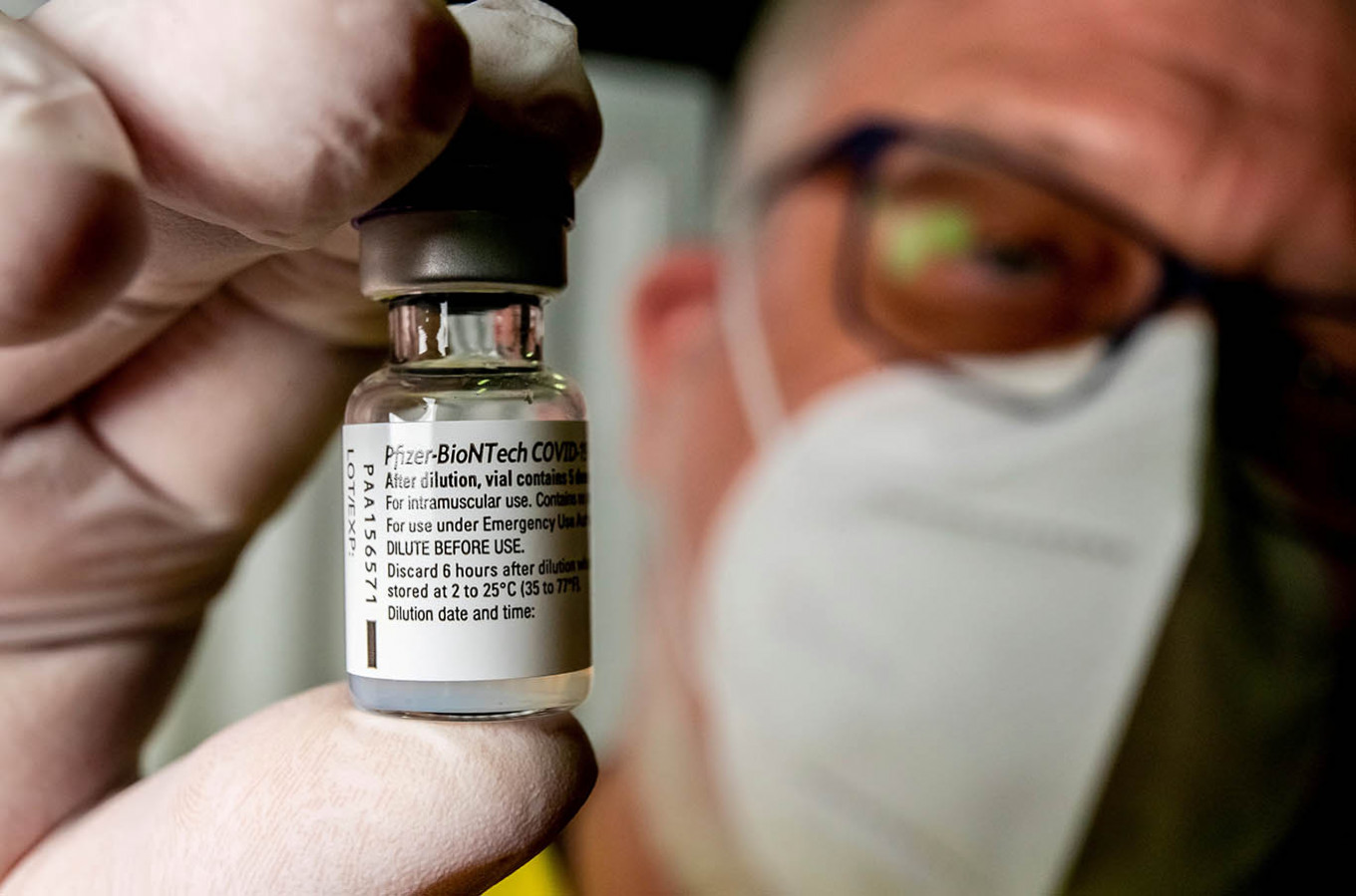
View all search resultsPfizer-BioNTech's Covid-flu jab disappoints in trial
The messenger RNA method made its debut with the Pfizer-BioNTech coronavirus vaccine, which was the first jab against Covid to be approved in the West in late 2020
Change text size
Gift Premium Articles
to Anyone
G
ermany's BioNTech and US pharma giant Pfizer said Friday they had suffered a setback in a late-stage trial of their combined mRNA vaccine against COVID-19 and influenza.
In a phase 3 trial, the combo vaccine showed a stronger immune response against the influenza A strain than against the less common influenza B strain.
Against COVID, the combo jab was as effective as the companies' standalone vaccine, they said.
The trial was carried out on more than 8,000 people aged 18-64.
Pfizer and BioNTech said they were "evaluating adjustments to the combination vaccine candidate aimed at improving immune responses against influenza B, and will discuss next steps with health authorities".
The disappointing result comes after rival mRNA vaccine maker Moderna in June said its combo flu and Covid jab had outperformed currently authorized standalone shots for the viruses in an advanced clinical trial.
The messenger RNA method made its debut with the Pfizer-BioNTech coronavirus vaccine, which was the first jab against COVID-19 to be approved in the West in late 2020.
Scientists believe mRNA vaccines, which provoke an immune response by delivering genetic molecules containing the code for key parts of a pathogen into human cells, could be a game-changer against many diseases.
They also take less time to develop than traditional vaccines.
BioNTech's COVID-19 shot was developed and approved by regulators in less than a year.
The German company is also working on mRNA-based vaccines against malaria and shingles, as well as mRNA-based cancer therapies.
The World Health Organization last year said it no longer considered COVID-19 a global health emergency, although the virus is still circulating.