Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
View all search resultsITB-produced ventilators for COVID-19 patients pass Health Ministry’s quality assurance
The ventilator, dubbed Vent-I, is designed primarily for COVID-19 patients who are still able to breathe independently.
Change text size
Gift Premium Articles
to Anyone
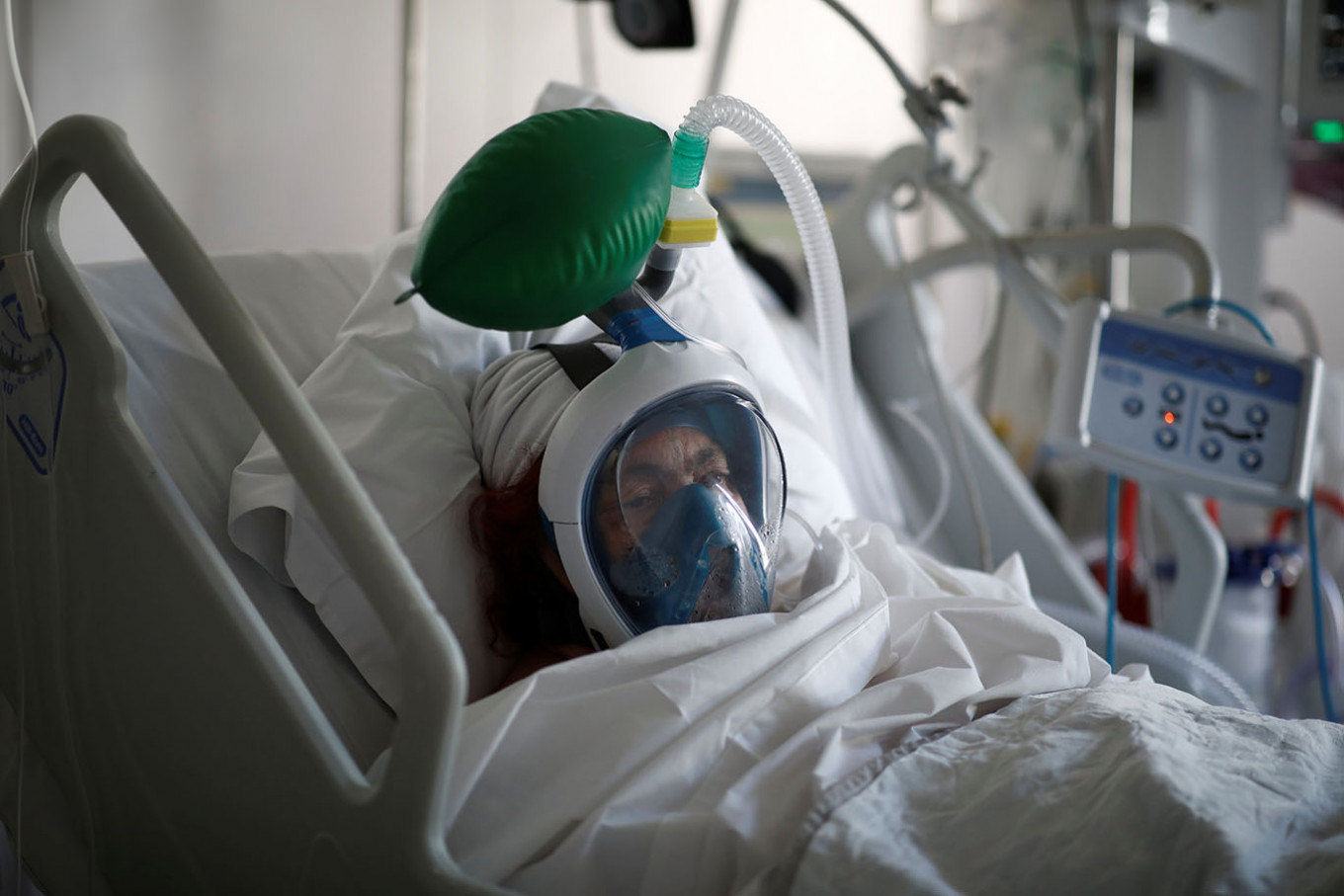 A patient suffering from COVID-19 wears a full-face Easybreath snorkeling mask given by sport chain Decathlon and turned into a ventilator for coronavirus treatment at the intensive care unit at Ambroise Pare clinic in Neuilly-sur-Seine near Paris, as the spread of the coronavirus disease continues in France, April 1. (REUTERS/Benoit Tessier)
A patient suffering from COVID-19 wears a full-face Easybreath snorkeling mask given by sport chain Decathlon and turned into a ventilator for coronavirus treatment at the intensive care unit at Ambroise Pare clinic in Neuilly-sur-Seine near Paris, as the spread of the coronavirus disease continues in France, April 1. (REUTERS/Benoit Tessier)
N
on-invasive ventilators produced by the Bandung Institute of Technology (ITB), in collaboration with Padjadjaran University Medical School in West Java, have passed quality assurance at the Health Ministry’s Health Research and Development Agency (Balitbangkes).
The new batch of ventilators is expected to ship soon to aid patients suffering from COVID-19 symptoms, including shortness of breath.
The ventilators, dubbed Vent-I, met the ministry’s general safety criteria on Tuesday for the continuous positive airway pressure (CPAP) systems that are being rapidly manufactured.
“It took at least a week to ensure that it passed the testing phase,” said Hari Tjahjono, a spokesperson for the Vent-I development team, on Wednesday.
Vent-I is designed primarily for COVID-19 patients who are still able to breathe independently, he said.
Read also: COVID-19: Weapons maker Pindad develops ventilators, protective gear
“So it’s not meant for intensive-care patients,” he said, adding that the development team had received technical assistance from Balitbangkes throughout the quality assurance for about two weeks.
After having been officially certified by the ministry, the ventilators will be immediately produced to help treat COVID-19 patients, Hari said. The development team received donations from an ITB-sponsored charity that will be used to accommodate the production of 300 to 500 Vent-I units, he said.
“It’s not exactly mass produced but rather charity produced,” Hari added.
The development team has also coordinated with the Association of Indonesian Anesthesiologists and Intensive Therapy Specialists (Perdatin) for the immediate distribution of the ventilators to a number of hospitals in West Java and East Java, according to him.
In regard to commercial use, Hari said he expected the market license to be issued by ITB within several days.
Read also: Raw materials coming from India to produce COVID-19 medicine
The need for ventilators has increasingly become urgent as the number of COVID-19 cases continues to increase across the country. Several stakeholders, both in the public and private sector, have since scrambled to produce a range of medical devices – including ventilators – to support the treatment of COVID-19 patients.
State-owned weapons manufacturer Pindad, for instance, developed a manual resuscitator ventilator and portable ventilator specifically designed for emergencies, dubbed the Pindad VRM and the Covent-20, respectively.
As of Wednesday, Indonesia has recorded 7,418 confirmed COVID-19 cases and 635 deaths linked to the disease. (rfa)