Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
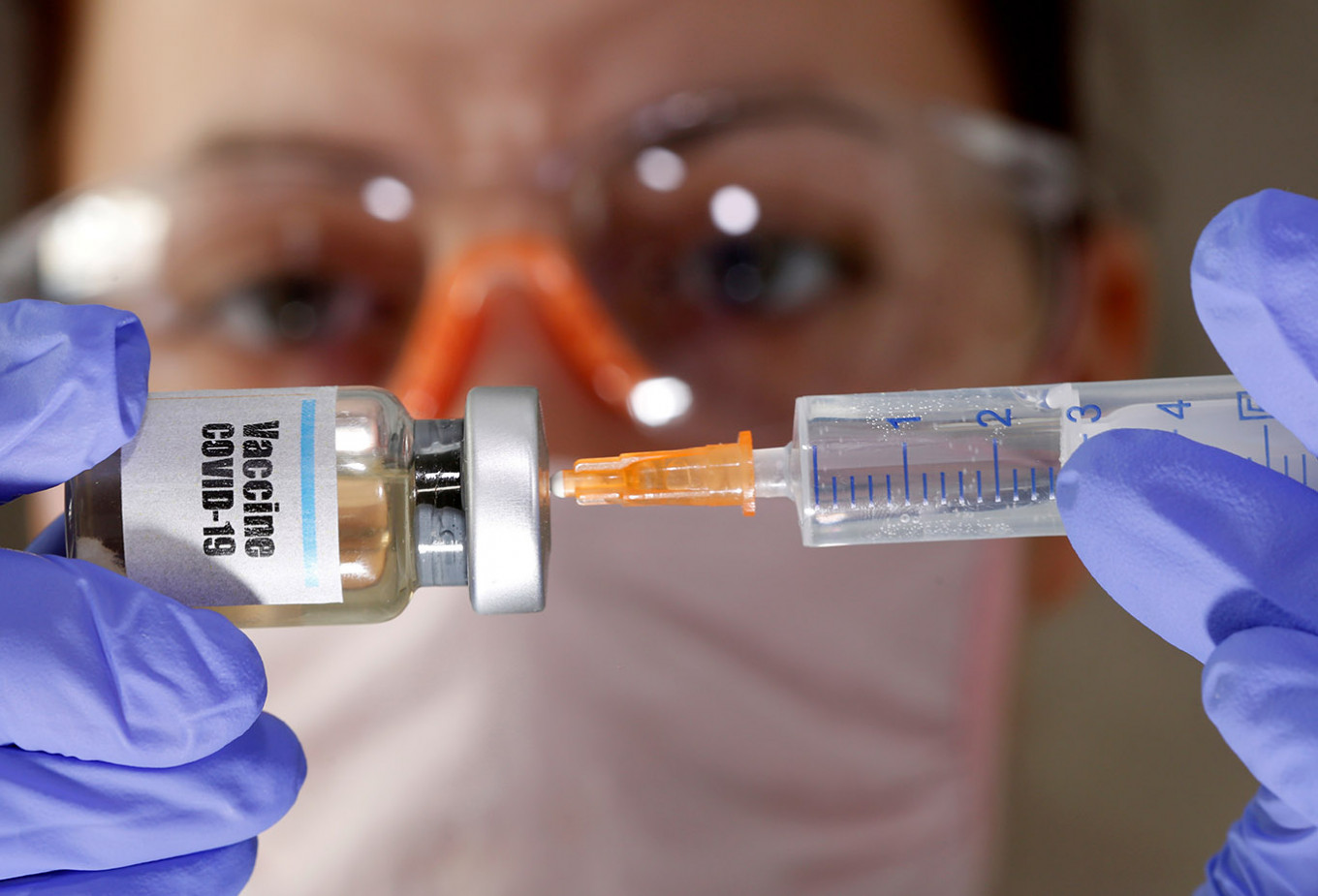
View all search resultsBio Farma to start pre-clinical trial for COVID-19 vaccine in 2021
Bio Farma will work on a COVID-19 vaccine under a consortium led by Eijkman Institute.
Change text size
Gift Premium Articles
to Anyone
State-owned pharmaceutical holding company PT Bio Farma is hoping to start a pre-clinical trial for a COVID-19 vaccine by 2021.
Bio Farma president director Honesti Basyir said during a hearing with the House of Representatives Commission VI overseeing trade, industry and state-owned enterprises (SOEs) that the company was working in a consortium led by Eijkman Institute to find a vaccine for the highly contagious coronavirus. Also in the consortium are the Health Research and Development Agency (Balitbangkes) and several universities.
“We expect to develop a seed vaccine by the end of 2020 so we can conduct a pre-clinical trial by the fourth quarter of 2021,” Honesti said during the virtual hearing.
The company also expected to start phase one of the clinical trial by the first quarter of 2022, he said.
COVID-19 had infected more than 7,400 people and killed 635 in Indonesia as of Wednesday afternoon, official data shows. To date, no vaccine or medicine has been developed.
Bio Farma is also seeking to work with the Bill Gates-backed Coalition for Epidemic Preparedness Innovations (CEPI), as well as with China-based vaccine producer Sinovac Biotech, to locally produce a vaccine in Indonesia.
“We have submitted a proposal to the CEPI to participate in the COVID-19 vaccine clinical trial and capacity building in Indonesia,” Honesti said, adding that the institution was currently reviewing the proposal.
Sinovac warmly welcomed the proposal and was working on the details of the collaboration with Bio Farma, he added.
Honesti gave assurances that the company would be ready to mass produce the vaccine once it is ready for distribution.
“Our production facility can handle up to 2 billion dosages, so it should be enough to produce the vaccine by ourselves.”