Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
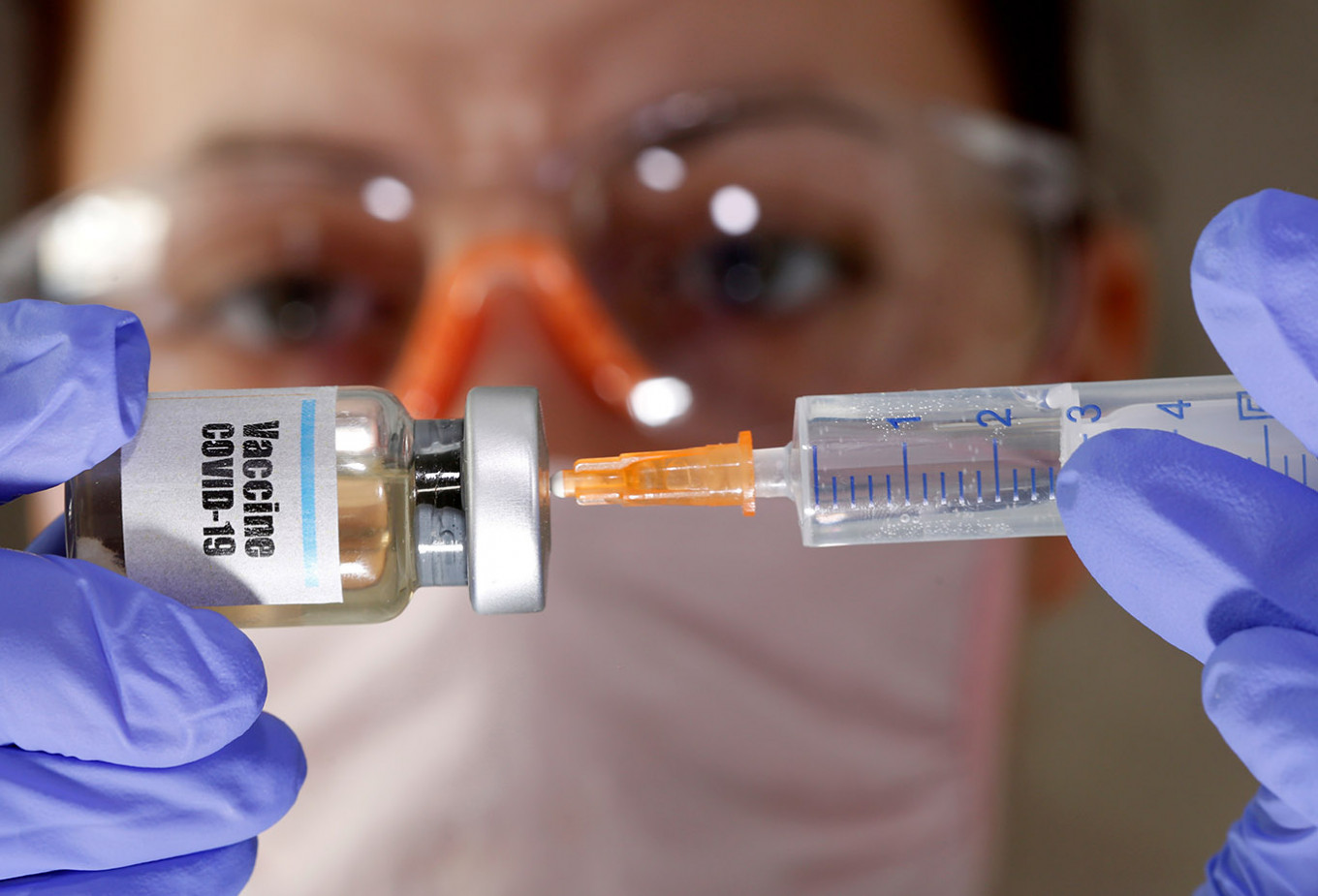
View all search resultsJokowi wants COVID-19 vaccine available within three months, research team says
Padjajaran University professor Kusnandi Rusmil said Jokowi had called on the research team to fast-track the potential vaccine during a private meeting held at the State Palace in Jakarta on Tuesday.
Change text size
Gift Premium Articles
to Anyone
P
resident Joko “Jokowi” Widodo has urged Padjajaran University’s Medical School research team in Bandung, West Java to make the Sinovac COVID-19 vaccine available for widespread use within three months amid mounting concerns over the ongoing health crisis.
Padjajaran University professor Kusnandi Rusmil said Jokowi had called on the research team to fast-track the potential vaccine during a private meeting held at the State Palace in Jakarta on Tuesday.
“There was a special instruction from the President to expedite [the production] of the coronavirus vaccine within three months, if possible,” Kusnandi told the press after the meeting.
However, he said the team could not possibly fulfill Jokowi’s request, as the clinical trials for the possible vaccine entailed a string of stringent protocols that would otherwise take years to complete under normal circumstances.
“We told [the President] that three months is impossible. We need to conduct proper testing,” Kusnandi added.
He went on to say that he expected the vaccine – developed by Chinese biopharmaceutical firm Sinovac Biotech in collaboration with state-owned pharmaceutical holding company PT Bio Farma – to pass phase III clinical trials as early as next January with a total of 1,620 samples tested.
Bio Farma president director Honesti Basyir said the company had planned to distribute 40 million doses of the vaccine per year as soon as the government authorized its widespread usage.
According to the official government count, Indonesia has 89,869 confirmed COVID-19 cases with 4,320 deaths as of Tuesday. (rfa)