Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
View all search resultsVaccination of elderly brings hope, but also caution
Authorities have greenlit the vaccination of elderly people as part of the state-led immunization drive against COVID-19.
Change text size
Gift Premium Articles
to Anyone
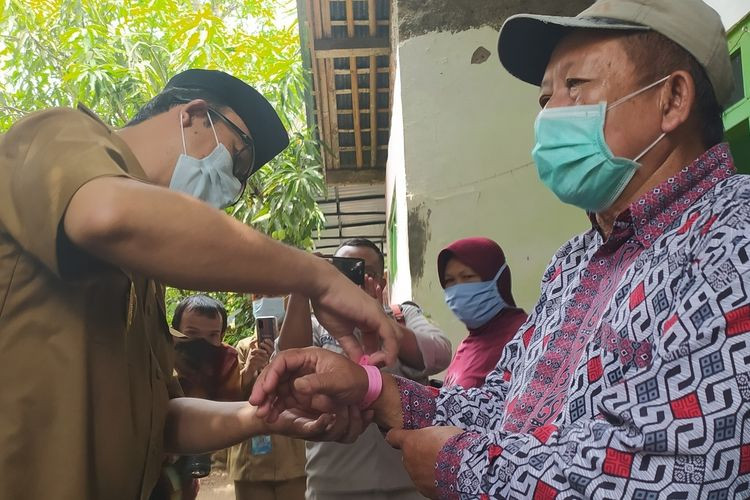 Banyumas Regent Achmad Husein puts a pink plastic bracelet on Edhi Ahmadi, 67, resident of Danaraja, Banyumas, East Java on Oct. 19, 2020. The Banyumas administration kick-started a pilot project dubbed Desa Jaga Komorbid, a derivative program of Central Java’s Jogo Tonggo (Taking Care of Neighbor) initiative. The project marks the elderly and people with comorbidities with pink plastic bracelets. (kompas.com/Fadlan Mukhtar Zain)
Banyumas Regent Achmad Husein puts a pink plastic bracelet on Edhi Ahmadi, 67, resident of Danaraja, Banyumas, East Java on Oct. 19, 2020. The Banyumas administration kick-started a pilot project dubbed Desa Jaga Komorbid, a derivative program of Central Java’s Jogo Tonggo (Taking Care of Neighbor) initiative. The project marks the elderly and people with comorbidities with pink plastic bracelets. (kompas.com/Fadlan Mukhtar Zain)
I
ndonesia has moved to authorize COVID-19 vaccinations for the elderly, bringing with it as much hope as caution with the limited availability of trial data for an age group most at risk from the pandemic due to underlying conditions.
The country's Food and Drug Monitoring Agency (BPOM) authorized the use of Chinese firm Sinovac Biotech's CoronaVac vaccine on the elderly, nearly a month after it issued a similar permit limited to people aged 18 to 59 years old.
For people 60 and over, the two shots of the vaccine are to be administered with a 28-day interval, as opposed to the 14 days for the younger population.
For the past month, the nation has focused its vaccine rollout on healthcare workers in the productive age bracket, even though there are also some 11,600 elderly medical workers working for and outside the pandemic response, Health Minister Budi Gunadi Sadikin has said.
Starting from Monday, workers over 59 years old are also able to receive the vaccine jabs.
"We'll start collecting data [of the elderly] outside medical workers for the inoculation of those over 60 years," the minister said on Sunday.
Read also: Data problems cast shadow over Indonesia's ambitious vaccine drive