Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
View all search resultsBumpy road for local vaccine development
Politicians continue to back Nusantara experimental vaccine
Change text size
Gift Premium Articles
to Anyone
Indonesia's bid to develop its own coronavirus vaccines either is marred with questionable trials backed by politicians or government plans to dissolve the research ministry that has helmed the country's vaccine consortium.
As part of its long-term strategy for self-sufficiency, the Research and Technology Ministry formed a vaccine consortium involving six institutions to develop the so-called Merah Putih vaccine, named after the country's flag colors. All of them are still conducting laboratory tests.
Separately, former health minister Terawan Agus Putranto initiated the development of another candidate vaccine last year after he issued a ministerial decree to appoint the Health Ministry as a sponsor, shortly before he was replaced as health minister. Other sponsors are American biotechnology firm AIVITA Biomedical and Indonesian pharmaceutical company PT Rama Emerald Multi Sukses.
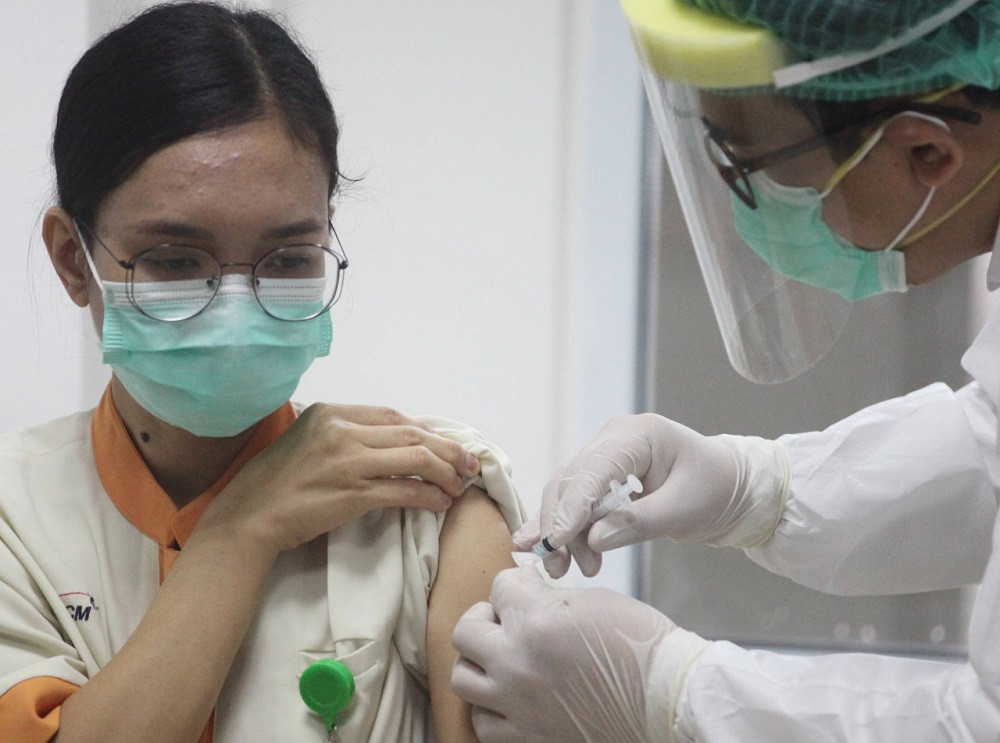
Politicians continue to back Terawan's experimental vaccine, dubbed Nusantara (archipelago) vaccine, by signing up as clinical trial volunteers despite red alerts from the Food and Drug Monitoring Agency (BPOM).
Scores of politicians and lawmakers, some of whom had previously demanded that the BPOM allow the Nusantara's mid-stage clinical trials to proceed, had their blood taken for research on the candidate vaccine at the Gatot Subroto Army Hospital, which used to be led by Terawan prior to his appointment as minister. The candidate vaccine employs a unique mechanism. It is tailored to each recipient through the use of their own antigen-presenting dendritic cells.
Political figures who willingly received the Nusantara candidate vaccine include business tycoon and former Golkar Party chairman Aburizal Bakrie as well as House of Representatives Deputy Speaker Sufmi Dasco Ahmad from the Gerindra Party. Some lawmakers at House Commission IX, which overseeing health, such as Emanuel Melkiades Laka Lena from Golkar and Saleh Daulay from the National Mandate Party (PAN), are also among them.
Aburizal is known as one of Terawan's patients for his "brain-cleaning" stroke treatment, which earned the latter sanctions from the Indonesian Doctors Association's (IDI) ethics council for the absence of clinically safe and effective evidence.
More lawmakers would volunteer in stages over the next few days, Melkiades told The Jakarta Post on Thursday.
The blood-drawing of voluntary research participants continues even after the BPOM, in its evaluation of Nusantara's clinical trials at Semarang-based Dr. Kariadi General Hospital, found the early data insufficient to proceed to the next phase.
"It's better for the research to be first developed in the pre-clinical phase before entering the clinical trials to get a certain basic concept, so that, upon clinical trials on humans, it won't turn into an uncertain trial," the BPOM said in a statement received by the Post on Wednesday.
Read also: Drug agency pressured to greenlight local vaccine
The agency said the experimental vaccine had yet to meet standards of manufacturing, laboratory and clinical practices. It had not been made in a sterile environment, nor was there a sufficient sterility assessment, which could potentially expose recipients to bacterial infection.
It also found that the subjects recruited included those who had already developed antibodies against the coronavirus and that Kariadi hospital’s ethics committee had never issued clearance for the trials nor monitored the safety of the subjects throughout the trials.
Around 71 percent of the subjects experienced undesirable events of mild or moderate degree after receiving the experimental vaccine. While three of the subjects saw an increase in antibody titers, eight saw a decline of their titers weeks after receiving the vaccine.
Other concerns raised by the agency are the need for importing the main components for making the vaccine from the United States, while AIVITA's Indonesian partner did not have the production facilities or biological products. Any technology transfer could take up to five years to develop in Indonesia, the agency said, while the process for Nusantara had not been entirely transparent. The agency also pointed to how American researchers from AIVITA, whose names were not recorded in the protocols, had been the ones answering evaluation questions instead of local researchers, although they did not present foreign researcher permits.
Gatot Subroto hospital head Budi Sulistya confirmed that the trials would proceed, saying on Thursday: "Procedures are important, but don't be shackled by procedures. The clinical trial stages are aligned with research protocols."
Melkiades dismissed the BPOM's evaluation, instead criticizing the agency for exercising "double standards and inconsistencies" when favoring imported vaccines.
Read also: Indonesian government promises big, then falters in vaccine procurement
Ines Atmosukarto, an Indonesian vaccine researcher working for an Australia-based biotech company, lambasted the move, calling it a very dangerous precedent that might not only put recipients at risk, but also Indonesia's name in research and development.
She expressed concerns over who would take responsibility should things go south, urging researchers to first address issues the BPOM has pointed out.
“No one seems to be talking about data from the [early] trials; what they talked about were issues irrelevant to innovation, such as [patriotism],” she told the Post.
Molecular biologist Ahmad Utomo called out the lack of trial data transparency from the vaccine development team, fearing as well the possibility of contamination.
“The country's funding is limited and we're currently developing the Merah Putih vaccine. Scientists would support fellow scientists who are working using accountable technology and data. So, prioritize funding for this vaccine instead,” he said.
Clinical pathologist Tonang Dwi Ardyanto said any lawmakers who had received COVID-19 jabs from imported brands should not be subjects in trials, as they had already developed antibodies. "If supporting local products is the main reason, then it's better to support the Merah Putih vaccine. The [platforms] used [in its development] are well known and commonly used.”
Read also: Ministry merger feared to jeopardize focus on science, research
Merah Putih researchers are spread across six institutions: the Eijkman Institute for Molecular Biology, Airlangga University (Unair), the Indonesian Institute of Sciences (LIPI), the University of Indonesia, the Bandung Institute of Technology and Gadjah Mada University.
They are currently developing seed vaccines in laboratories before eventually handing them over to the pharmaceutical companies that would run clinical trials and manufacturing. The platforms of candidate vaccines currently worked on vary from inactivated virus to mRNA platforms.
Research and Technology Minister Bambang Bodjonegoro, who also heads the National Research Agency (BRIN), said Eijkman and Unair had progressed rapidly so far, as Eijkman would partner with state-owned firm Bio Farma and Unair was in early talks with local firm Biotis Pharmaceuticals. Eijkman was expected to hand its seed vaccines to Bio Farma in May, he said. Other institutions had yet to find partnering companies.
"We have to admit that we don't have any experience in developing vaccines from zero, starting from making the seed vaccines to manufacturing, until rollout," Bambang said on Tuesday, highlighting the need to upscale the pharmaceutical industry's capacity.
Read also: Private sector picks up slack in vaccination drive
Health Minister Budi Gunadi Sadikin said Indonesia would need to be self-resilient amid limited vaccine supplies and border restrictions. The ministry was allocating Rp 400 billion for vaccine development this year, Budi said on Tuesday.
But President Joko "Jokowi'' Widodo now plans to dissolve the research ministry by merging it with the education ministry to make way for a new investment ministry as well as to turn BRIN into an independent state agency. Experts have voiced concerns of possible stalling and politicization of ongoing and future research.
LIPI biotechnology researcher Wien Kusharyoto, who is involved in the Merah Putih vaccine development, said the consortium should be maintained and issues on the split should not affect administrative affairs that could hinder funding.