Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
View all search resultsWhich drugs are effective for COVID-19?
Many reports are circulating about possible drugs, causing confusion among the public.
Change text size
Gift Premium Articles
to Anyone
T
he COVID-19 pandemic has spread to 210 countries. As of April 30, more than 3.2 million people had tested positive for the virus and 228,239 people had died.
Many reports are circulating about possible drugs, causing confusion among the public.
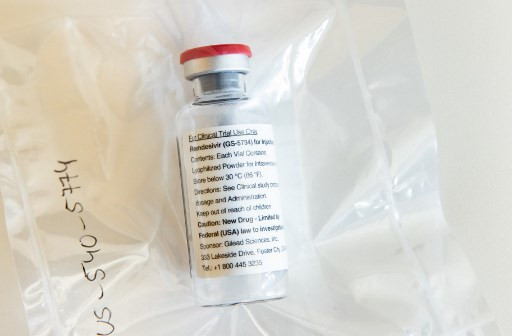
Several types of drugs are being studied to treat COVID-19. Most of the drugs are antiviral drugs that have previously been used for other conditions. Among the antiviral medications are favipiravir, or Avigan, which was used to treat influenza in Japan; remdesivir, which was tested on Ebola; ritonavir-lopinavir, an antiviral drug given to HIV patients; and ribavirin which is used for hepatitis C and other viral hemorrhagic fevers.
The Food and Drug Monitoring Agency (BPOM) has released its recommendations for COVID-19 therapy in Indonesia. In Indonesia, the antiviral therapy to be given to patients with moderate and severe symptoms is favipiravir.
Other countries and the World Health Organization have not yet released specific guidelines on COVID-19 drug treatments, but we can see that various antiviral medications are being used in other countries. The most common are favipiravir, remdesivir, lopinavir-ritonavir and ribavirin.
Favipiravir and remdesivir work against viruses by inhibiting the RNA-dependent RNA polymerase (RdRP), an enzyme needed by viruses to replicate their genetic material in human cells. Ribavirin also works by inhibiting RdRP as well as interfering with the replication of viral genetic material in other ways. In addition, ribavirin was also shown to increase the immunity of human cells to viruses.
Lopinavir-ritonavir was found to hinder the activity of a type of protease needed by viruses to replicate.
The replication of viruses is directly related to the severity of the disease. Data suggests that a higher SARS-CoV-2 viral load – a measure of the amount of the virus in a patient’s body – is related to more severe symptoms. Therefore, the more we can inhibit viral replication and cause a lower viral load, the more we can hopefully alleviate the severity of the disease.
Favipiravir, remdesivir, lopinavir-ritonavir and ribavirin have demonstrated effectiveness against SARS-CoV-2 in vitro – meaning in laboratory tests using cell cultures. To date, there is no strong evidence of any drugs being effective against the virus in humans, regardless of the few case studies claiming treatments were effective on patients.
Several ongoing clinical trials of the antiviral medications are expected to conclude at the end of the year. Only then can we obtain more evidence about which drugs, if any, effectively treat COVID-19.
Drugs used to treat other diseases are also being studied to treat COVID-19. These are chloroquine (CQ) and hydroxychloroquine (HCQ). These repurposed drugs have been used to treat malaria and chronic inflammatory diseases. HCQ is a derivate of CQ with fewer toxic effects than CQ.
Read also: Indonesia rallies to keep COVID-19 vaccines, drugs affordable
In vitro studies suggest that both CQ and HCQ are efficient at inhibiting SARS- CoV-2. Both drugs prevent the virus’ entry and modulate the immune system. Clinical trial data from both drugs is still limited and inconclusive given small sample sizes with poorly controlled or uncontrolled clinical trials. Numerous randomized control trials are ongoing to establish the effectiveness of these drugs on COVID-19. Despite lacking convincing evidence to support the use of CQ, China’s medical advisory board has suggested its inclusion in treatment guidelines, making CQ the first drug used in China and overseas to treat moderate to severe cases of COVID-19.
Type-1 interferons (IFNs) are another potential candidate. IFNs are normally produced by our body in response to viral infections. Therefore, IFNs have general antiviral properties. Previously, IFNs were investigated for the treatment of the Middle East Respiratory Syndrome (MERS).
The MERS virus belongs to the same genus as SARS-CoV-2: betacoronavirus. The close relationship between these viruses is the basis for the use of IFNs to treat COVID-19. Although current Chinese guidelines list interferons as an alternative for combination therapy, the research results are conflicting and no clinical trials have been completed so far.
Monoclonal antibodies against interleukin-6, a protein produced by various cells to trigger inflammation, have also been used as adjunctive therapy in China and Singapore.
Known as tocilizumab, these antibodies are administered to alleviate the “cytokine storm” that causes profound damage to the lungs and other organs in COVID-19 patients. In several case reports, this treatment was found to be successful on COVID-19 patients experiencing severe forms of the illness. One small study with no control reported clinical improvement in 91 percent of patients. Further clinical studies are ongoing to confirm this finding.
Due to the lack of clinical trials on these drugs, they can only be given to COVID-19 patients under close medical supervision.
Another treatment uses convalescent plasma: plasma extracted from the blood of recovered COVID-19 patients. This plasma contains antibodies that neutralize SARS-CoV-2.
An early study showed that a single dose of 200 milliliters of convalescent plasma given to COVID-19 patients with severe forms of the illness showed promising results. Therefore, a large clinical trial in the United States is underway. The Food and Drug Administration is encouraging recovered patients to donate their blood to support the study. The actor Tom Hanks has reportedly offered to donate his blood plasma following his recovery from COVID-19.
The above information is important for people to know and understand so they can sort out the information available. Not all of what is circulated is true, and websites may exaggerate information with a catchy title. For a drug to be approved for use on humans, the drug needs to undergo rigorous testing through in-vitro studies, pre-clinical trials using animals and clinical studies to demonstrate its safety and efficacy.
Lecturers in the Pharmacy Studies program at the Indonesia International Institute for Life Sciences (i3L)