Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
View all search resultsIndia orders drugmakers to meet global standards by January after cough syrup deaths
The government had directed drugmakers in late 2023 to upgrade plants to norms recommended by the World Health Organization (WHO), including measures to prevent cross-contamination and enable batch testing.
Change text size
Gift Premium Articles
to Anyone
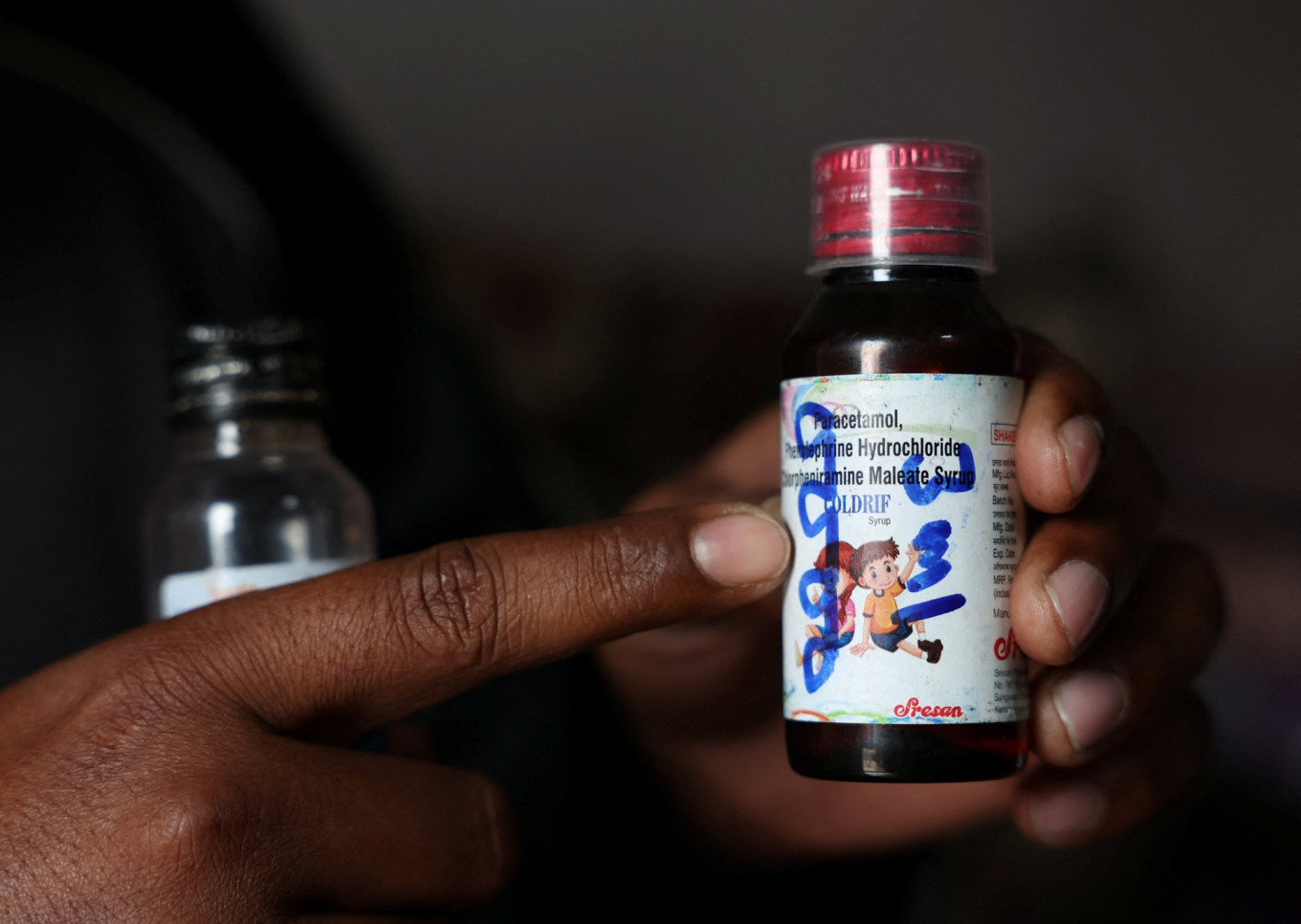 Saddam Mansuri shows a bottle of Coldrif cough syrup, which has been linked to the deaths of multiple children and which he had been giving to his one-year-old child, in Parasia, Madhya Pradesh, India, on Oct. 10, 2025. Coldrif cough syrup has been banned and is being recovered by community healthcare workers amid a door-to-door campaign to spread awareness and recall them. (Reuters/Priyanshu Singh)
Saddam Mansuri shows a bottle of Coldrif cough syrup, which has been linked to the deaths of multiple children and which he had been giving to his one-year-old child, in Parasia, Madhya Pradesh, India, on Oct. 10, 2025. Coldrif cough syrup has been banned and is being recovered by community healthcare workers amid a door-to-door campaign to spread awareness and recall them. (Reuters/Priyanshu Singh)
I
ndia's drug regulator on Friday ordered state authorities to ensure all pharmaceutical factories comply with international manufacturing standards by January, rejecting industry pleas for more time after toxic cough syrup was linked to additional child deaths since September.
The government had directed drugmakers in late 2023 to upgrade plants to norms recommended by the World Health Organization (WHO), including measures to prevent cross-contamination and enable batch testing.
The move followed global outrage after India-made cough syrups were tied to the deaths of more than 140 children in Africa and Central Asia, tarnishing the country’s reputation as the "pharmacy of the world."
Large pharmaceutical firms met a June 2024 deadline, while smaller manufacturers were given until December 2025 as part of an earlier extension. Industry groups had sought further extensions, warning that compliance costs could bankrupt small businesses.
But the deaths of 24 children in central India linked to contaminated syrup prompted authorities to hold firm.
The Central Drugs Standard Control Organisation said in a notice, seen by Reuters, that the revised standards under "Schedule M" will apply to all manufacturers from Jan. 1, 2026, and directed state regulators to begin inspections.
“In case any manufacturing unit is found non-complying with the requirements of revised Schedule M during inspections, strict action shall be initiated,” the notice, signed by Drugs Controller General Rajeev Singh Raghuvanshi, said.
Firms that do not fall under the extension criteria should be inspected immediately, the notice said.
He asked states to treat the matter as a "top priority".
Raghuvanshi's office did not immediately respond to a request for a comment.
Reuters reported exclusively on Oct. 17 that no extension would be granted.
However, an association representing small- and medium-sized drugmakers warned the mandate could shutter many factories, causing job losses and pushing up medicine prices.
“What good is quality if there is no affordability?” said Jagdeep Singh, secretary of the SME Pharma Industries Confederation.








