Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
View all search resultsSiloam Hospitals in Indonesia now provide COVID-19 serology tests
The serology test is designed to determine whether a patient has been exposed to COVID-19 and whether they have developed antibodies against the virus.
Change text size
Gift Premium Articles
to Anyone
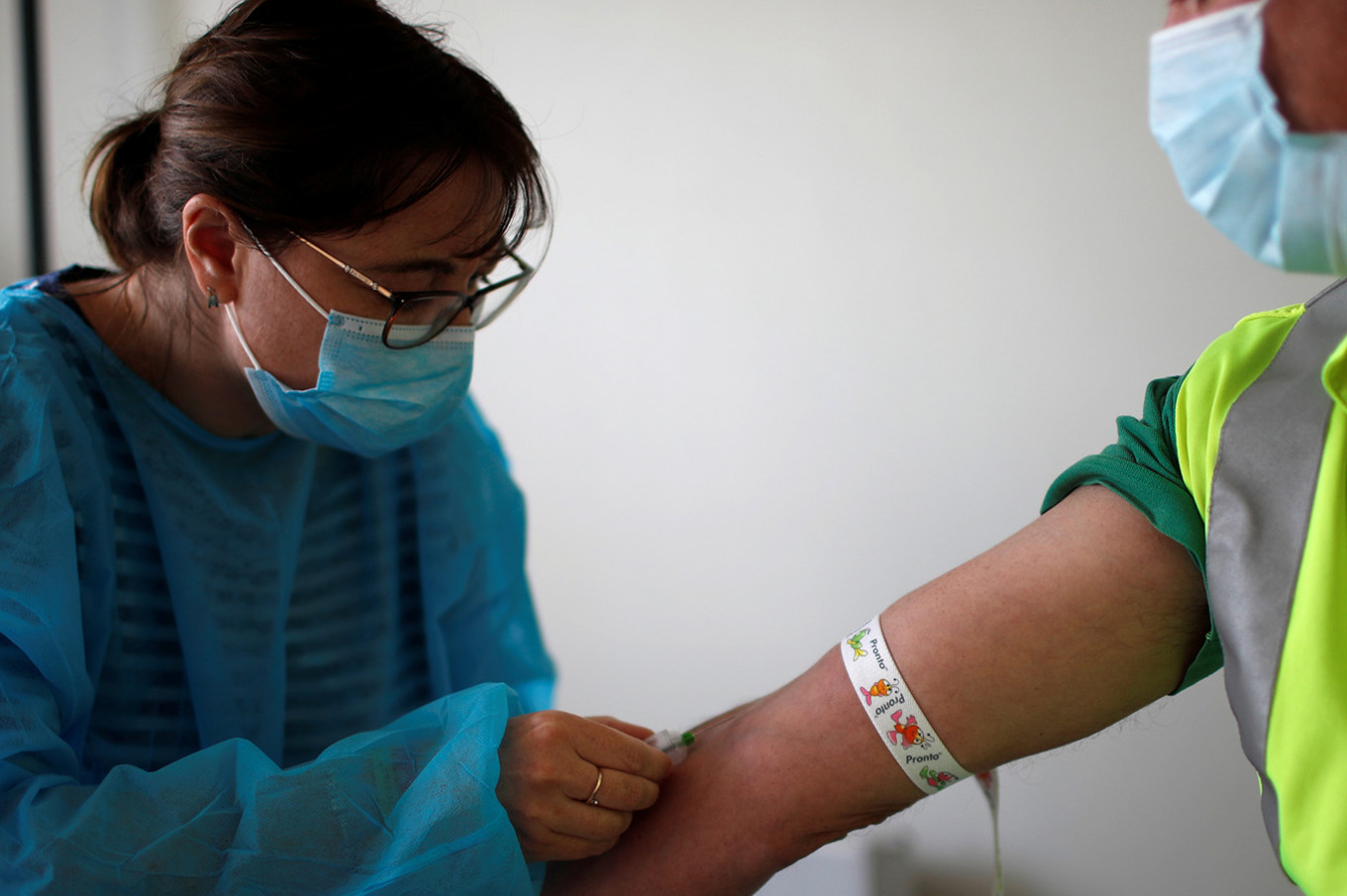 A medical biologist, wearing a protective suit and face mask, takes a blood sample from a garbage collector for a serological test during a COVID-19 testing operation at Veolia waste collection site in Saint-Denis, Paris during the outbreak of COVID-19 in France on May 7, 2020. (REUTERS/Gonzalo Fuentes)
A medical biologist, wearing a protective suit and face mask, takes a blood sample from a garbage collector for a serological test during a COVID-19 testing operation at Veolia waste collection site in Saint-Denis, Paris during the outbreak of COVID-19 in France on May 7, 2020. (REUTERS/Gonzalo Fuentes)
H
ospital operator Siloam Hospitals Group has launched its latest COVID-19 test known as the Elecsys Anti-SARS-CoV-2 serology test developed by Swiss multinational healthcare company Roche.
"There is a high demand for faster, more affordable tests and bigger testing capacity within the 'new normal' scheme in Indonesia," the group's deputy president director Caroline Riady said in an online media briefing on Wednesday.
The serology test -- now available in all Siloam hospitals in Indonesia -- is designed to determine whether a patient has been exposed to COVID-19 and whether they have developed antibodies against the virus.
This antibody testing is considered crucial to better understand the true scope of the pandemic.
Caroline said each serology test cost Rp 199,000 (US$14) per person, cheaper than rapid COVID-19 testing or polymerase chain reaction (PCR) tests.
“Both the serology test and rapid test detect antibodies against SARS-CoV-2. However, the serology test is a laboratory test conducted by robotic instruments with more accurate and objective results,” she added.
Read also: Indonesia records spike in COVID-19 cases as govt eases restrictions
Roche Diagnostics Indonesia country manager Ahmed Hassan claimed the test had 100 percent sensitivity with no false-negative results. Based on an assessment of 5,272 samples, the serology test has 99.81 percent specificity with a low chance of false positive.
It also claimed to be able to detect antibodies with 100 percent sensitivity in samples taken 14 days after a PCR-confirmed infection.
"It takes 18 to 20 minutes from the sample being inserted into the instrument until the results come out," Ahmed said, “The capacity is 86 to 170 tests per hour depending on the instrument throughput,”.
Ahmed added that Roche’s Elecsys serology test had recently won emergency use clearance from the United States Food and Drug Administration (FDA). The product has also been approved by Public Health England in the United Kingdom and has been widely used in Singapore.
As of Wednesday, Indonesia has recorded 34,316 confirmed COVID-19 cases with 1,959 fatalities, according to the government's official count.
The country has repeatedly been criticized for having one of the lowest test rates in the world. As of Wednesday noon, Indonesia has tested 287,478 people, which means it has conducted 1,053 tests per 1 million people. In comparison, Malaysia has conducted 19,120 tests per 1 million people, while Singapore has conducted 83,571.
Previously, Research and Technology Minister Bambang Brodjonegoro said the Indonesian Institute of Science (LIPI) was currently in the process of developing more accurate test kits using the reverse transcription loop-mediated isothermal amplification (RT-LAMP) method, which is expected to be ready by July.









