Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
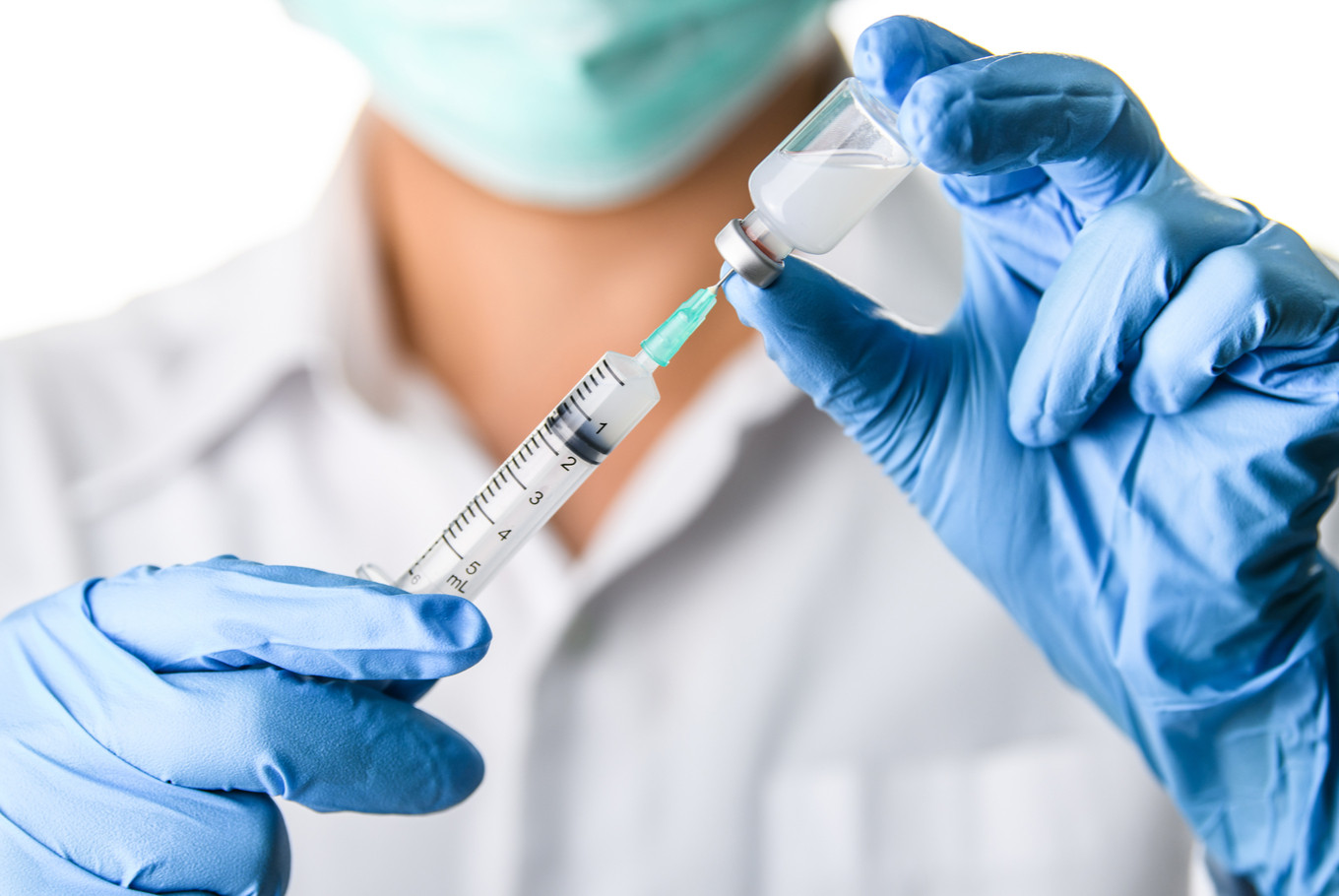
View all search resultsCanSino to start Phase III trial of COVID-19 vaccine in Saudi
Change text size
Gift Premium Articles
to Anyone
S
audi Arabia will soon begin Phase III clinical trials on around 5,000 people for a COVID-19 vaccine developed by China's CanSino Biologics Inc, a Saudi health ministry spokesman said on Sunday.
Last month, CanSino's co-founder said the company was in talks with Russia, Brazil, Chile and Saudi Arabia to launch a Phase III trial of the vaccine candidate, Ad5-nCOV.
The vaccine uses a harmless cold virus known as adenovirus type-5 (Ad5) to carry genetic material from the coronavirus into the body.
Researchers said last month that CanSino's vaccine, co-developed with China's military research unit, appeared to be safe and induced immune responses in most subjects.
Saudi Arabia plans to test the vaccine alongside a placebo on 5,000 volunteers and is currently preparing trials in the cities of Riyadh, Dammam and Mecca, Saudi state news agency SPA said on Saturday.
No COVID-19 vaccine has been approved for commercial use.
CanSino's candidate became the first in China to move into human testing in March but other potential vaccines developed by Sinovac Biotech and a unit of China National Pharmaceutical Group (Sinopharm) have already been approved for Phase III trials overseas.