Popular Reads
Top Results
Can't find what you're looking for?
View all search resultsPopular Reads
Top Results
Can't find what you're looking for?
View all search resultsPfizer says its COVID-19 jab safe for children aged 5-11
The vaccine would be administered at a lower dosage than for people over 12, they said.
Change text size
Gift Premium Articles
to Anyone
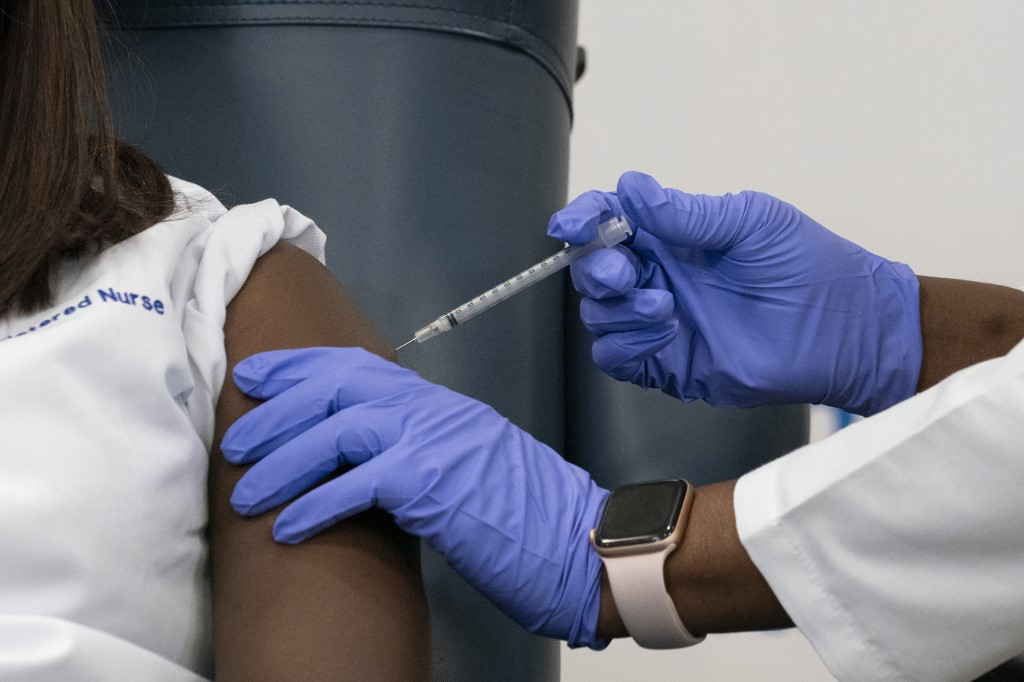 Sandra Lindsay(L), a nurse at Long Island Jewish Medical Center, is inoculated with the Covid-19 vaccine by Dr. Michelle Chester, at Long Island Jewish Medical Center, on December 14, 2020 in the Queens borough of New York. - The rollout of the Pfizer and BioNTech vaccine, the first to be approved by the Food and Drug Administration, ushers in the biggest vaccination effort in US history. More than 299,000 Americans have been killed by the virus, including over 35,000 residents of New York state. (AFP/Mark Lennihan / POOL)
Sandra Lindsay(L), a nurse at Long Island Jewish Medical Center, is inoculated with the Covid-19 vaccine by Dr. Michelle Chester, at Long Island Jewish Medical Center, on December 14, 2020 in the Queens borough of New York. - The rollout of the Pfizer and BioNTech vaccine, the first to be approved by the Food and Drug Administration, ushers in the biggest vaccination effort in US history. More than 299,000 Americans have been killed by the virus, including over 35,000 residents of New York state. (AFP/Mark Lennihan / POOL)
P
fizer and BioNTech on Monday said trial results showed their coronavirus vaccine is safe and produces a robust immune response in children aged five to 11, adding that they would seek regulatory approval shortly.
The vaccine would be administered at a lower dosage than for people over 12, they said.
"In participants five to 11 years of age, the vaccine was safe, well tolerated and showed robust neutralising antibody responses," US giant Pfizer and its German partner said in a joint statement.
They plan to submit their data to regulatory bodies in the European Union, the United States and around the world "as soon as possible".
Ashish Jha, dean of Brown University School of Public Health and a leading Covid expert in the US, called it the "good news" many parents had been waiting for.
If all goes well and approval follows, "my 9-year will get a shot by Halloween!" he tweeted.
The trial results are the first of their kind for children under 12, with a Moderna trial for 6-11 year olds still ongoing.